Huntingtin regulates mammary stem cell division and differentiation
- PMID: 24749073
- PMCID: PMC3986500
- DOI: 10.1016/j.stemcr.2014.02.011
Huntingtin regulates mammary stem cell division and differentiation
Abstract
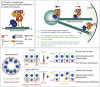
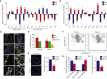
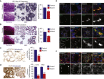
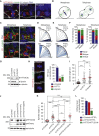
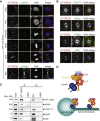
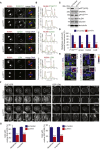
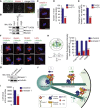
Little is known about the mechanisms of mitotic spindle orientation during mammary gland morphogenesis. Here, we report the presence of huntingtin, the protein mutated in Huntington's disease, in mouse mammary basal and luminal cells throughout mammogenesis. Keratin 5-driven depletion of huntingtin results in a decreased pool and specification of basal and luminal progenitors, and altered mammary morphogenesis. Analysis of mitosis in huntingtin-depleted basal progenitors reveals mitotic spindle misorientation. In mammary cell culture, huntingtin regulates spindle orientation in a dynein-dependent manner. Huntingtin is targeted to spindle poles through its interaction with dynein and promotes the accumulation of NUMA and LGN. Huntingtin is also essential for the cortical localization of dynein, dynactin, NUMA, and LGN by regulating their kinesin 1-dependent trafficking along astral microtubules. We thus suggest that huntingtin is a component of the pathway regulating the orientation of mammary stem cell division, with potential implications for their self-renewal and differentiation properties.
Figures

Similar articles
-
Mitotic spindle: focus on the function of huntingtin.Int J Biochem Cell Biol. 2011 Jun;43(6):852-6. doi: 10.1016/j.biocel.2011.03.009. Epub 2011 Mar 23. Int J Biochem Cell Biol. 2011. PMID: 21439401 Review.
-
Multifunctional protein 4.1R regulates the asymmetric segregation of Numb during terminal erythroid maturation.J Biol Chem. 2021 Sep;297(3):101051. doi: 10.1016/j.jbc.2021.101051. Epub 2021 Aug 6. J Biol Chem. 2021. PMID: 34364872 Free PMC article.
-
NuMA localization, stability, and function in spindle orientation involve 4.1 and Cdk1 interactions.Mol Biol Cell. 2013 Dec;24(23):3651-62. doi: 10.1091/mbc.E13-05-0277. Epub 2013 Oct 9. Mol Biol Cell. 2013. PMID: 24109598 Free PMC article.
-
Evidence for dynein and astral microtubule-mediated cortical release and transport of Gαi/LGN/NuMA complex in mitotic cells.Mol Biol Cell. 2013 Apr;24(7):901-13. doi: 10.1091/mbc.E12-06-0458. Epub 2013 Feb 6. Mol Biol Cell. 2013. PMID: 23389635 Free PMC article.
-
Role of NuMA in vertebrate cells: review of an intriguing multifunctional protein.Front Biosci. 2006 Jan 1;11:1137-46. doi: 10.2741/1868. Front Biosci. 2006. PMID: 16146802 Review.
Cited by
-
Mitochondrial Abnormalities and Synaptic Damage in Huntington's Disease: a Focus on Defective Mitophagy and Mitochondria-Targeted Therapeutics.Mol Neurobiol. 2021 Dec;58(12):6350-6377. doi: 10.1007/s12035-021-02556-x. Epub 2021 Sep 14. Mol Neurobiol. 2021. PMID: 34519969 Review.
-
Regulating the regulator: Numb acts upstream of p53 to control mammary stem and progenitor cell.J Cell Biol. 2015 Nov 23;211(4):737-9. doi: 10.1083/jcb.201510104. J Cell Biol. 2015. PMID: 26598611 Free PMC article.
-
Satellite Cells in Muscular Dystrophy - Lost in Polarity.Trends Mol Med. 2016 Jun;22(6):479-496. doi: 10.1016/j.molmed.2016.04.002. Epub 2016 May 5. Trends Mol Med. 2016. PMID: 27161598 Free PMC article. Review.
-
Gene targeting techniques for Huntington's disease.Ageing Res Rev. 2021 Sep;70:101385. doi: 10.1016/j.arr.2021.101385. Epub 2021 Jun 5. Ageing Res Rev. 2021. PMID: 34098113 Free PMC article. Review.
-
Molecular mechanisms of asymmetric divisions in mammary stem cells.EMBO Rep. 2016 Dec;17(12):1700-1720. doi: 10.15252/embr.201643021. Epub 2016 Nov 21. EMBO Rep. 2016. PMID: 27872203 Free PMC article. Review.
References
-
- Asselin-Labat M.L., Vaillant F., Sheridan J.M., Pal B., Wu D., Simpson E.R., Yasuda H., Smyth G.K., Martin T.J., Lindeman G.J., Visvader J.E. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465:798–802. - PubMed
-
- Bolte S., Cordelières F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006;224:213–232. - PubMed
-
- Bouras T., Pal B., Vaillant F., Harburg G., Asselin-Labat M.L., Oakes S.R., Lindeman G.J., Visvader J.E. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell. 2008;3:429–441. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

