The role of pericytes in neurovascular unit remodeling in brain disorders
- PMID: 24743889
- PMCID: PMC4013640
- DOI: 10.3390/ijms15046453
The role of pericytes in neurovascular unit remodeling in brain disorders
Abstract
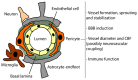
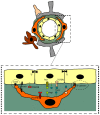
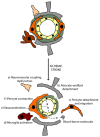
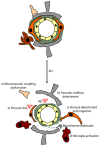
Neurons are extremely vulnerable cells that tightly rely on the brain's highly dynamic and complex vascular network that assures an accurate and adequate distribution of nutrients and oxygen. The neurovascular unit (NVU) couples neuronal activity to vascular function, controls brain homeostasis, and maintains an optimal brain microenvironment adequate for neuronal survival by adjusting blood-brain barrier (BBB) parameters based on brain needs. The NVU is a heterogeneous structure constituted by different cell types that includes pericytes. Pericytes are localized at the abluminal side of brain microvessels and contribute to NVU function. Pericytes play essential roles in the development and maturation of the neurovascular system during embryogenesis and stability during adulthood. Initially, pericytes were described as contractile cells involved in controlling neurovascular tone. However, recent reports have shown that pericytes dynamically respond to stress induced by injury upon brain diseases, by chemically and physically communicating with neighboring cells, by their immune properties and by their potential pluripotent nature within the neurovascular niche. As such, in this paper, we would like to review the role of pericytes in NVU remodeling, and their potential as targets for NVU repair strategies and consequently neuroprotection in two pathophysiologically distinct brain disorders: ischemic stroke and Alzheimer's disease (AD).
Figures
Similar articles
-
Pericytes of the neurovascular unit: key functions and signaling pathways.Nat Neurosci. 2016 May 26;19(6):771-83. doi: 10.1038/nn.4288. Nat Neurosci. 2016. PMID: 27227366 Free PMC article. Review.
-
The Translational Significance of the Neurovascular Unit.J Biol Chem. 2017 Jan 20;292(3):762-770. doi: 10.1074/jbc.R116.760215. Epub 2016 Dec 5. J Biol Chem. 2017. PMID: 27920202 Free PMC article. Review.
-
Microglia-Mediated Neurovascular Unit Dysfunction in Alzheimer's Disease.J Alzheimers Dis. 2023;94(s1):S335-S354. doi: 10.3233/JAD-221064. J Alzheimers Dis. 2023. PMID: 36683511 Free PMC article. Review.
-
An isogenic neurovascular unit model comprised of human induced pluripotent stem cell-derived brain microvascular endothelial cells, pericytes, astrocytes, and neurons.Fluids Barriers CNS. 2019 Aug 7;16(1):25. doi: 10.1186/s12987-019-0145-6. Fluids Barriers CNS. 2019. PMID: 31387594 Free PMC article.
-
The abluminal endothelial membrane in neurovascular remodeling in health and disease.Sci Signal. 2012 Aug 7;5(236):re4. doi: 10.1126/scisignal.2002886. Sci Signal. 2012. PMID: 22871611 Review.
Cited by
-
The Science of Vascular Contributions to Cognitive Impairment and Dementia (VCID): A Framework for Advancing Research Priorities in the Cerebrovascular Biology of Cognitive Decline.Cell Mol Neurobiol. 2016 Mar;36(2):281-8. doi: 10.1007/s10571-016-0334-7. Epub 2016 Apr 19. Cell Mol Neurobiol. 2016. PMID: 27095366 Free PMC article. Review.
-
Pericytes in Brain Injury and Repair After Ischemic Stroke.Transl Stroke Res. 2017 Apr;8(2):107-121. doi: 10.1007/s12975-016-0504-4. Epub 2016 Nov 12. Transl Stroke Res. 2017. PMID: 27837475 Free PMC article. Review.
-
The Role of Pericytes in Ischemic Stroke: Fom Cellular Functions to Therapeutic Targets.Front Mol Neurosci. 2022 Apr 13;15:866700. doi: 10.3389/fnmol.2022.866700. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35493333 Free PMC article. Review.
-
Alteration in the Function and Expression of SLC and ABC Transporters in the Neurovascular Unit in Alzheimer's Disease and the Clinical Significance.Aging Dis. 2020 Mar 9;11(2):390-404. doi: 10.14336/AD.2019.0519. eCollection 2020 Apr. Aging Dis. 2020. PMID: 32257549 Free PMC article. Review.
-
What Are the Roles of Pericytes in the Neurovascular Unit and Its Disorders?Neurology. 2023 May 16;100(20):970-977. doi: 10.1212/WNL.0000000000207379. Neurology. 2023. PMID: 37188542 Free PMC article. No abstract available.
References
-
- Zlokovic B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. - PubMed
-
- Ruitenberg A., den Heijer T., Bakker S.L., van Swieten J.C., Koudstaal P.J., Hofman A., Breteler M.M. Cerebral hypoperfusion and clinical onset of dementia: The Rotterdam study. Ann. Neurol. 2005;57:789–794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

