Glutathione protects brain endothelial cells from hydrogen peroxide-induced oxidative stress by increasing nrf2 expression
- PMID: 24737944
- PMCID: PMC3984961
- DOI: 10.5607/en.2014.23.1.93
Glutathione protects brain endothelial cells from hydrogen peroxide-induced oxidative stress by increasing nrf2 expression
Abstract
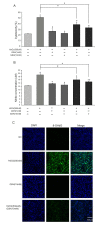
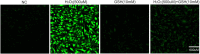
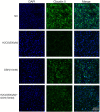
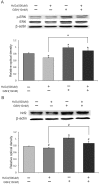
Glutathione (GSH) protects cells against oxidative stress by playing an antioxidant role. Protecting brain endothelial cells under oxidative stress is key to treating cerebrovascular diseases and neurodegenerative diseases including Alzheimer's disease and Huntington's disease. In present study, we investigated the protective effect of GSH on brain endothelial cells against hydrogen peroxide (H2O2). We showed that GSH attenuates H2O2-induced production of nitric oxide (NO), reactive oxygen species (ROS), and 8-Oxo-2'-deoxyguanosine (8-OHdG), an oxidized form of deoxiguanosine. GSH also prevents H2O2-induced reduction of tight junction proteins. Finally, GSH increases the level of nuclear factor erythroid 2-related factor 2 (Nrf2) and activates Nrf2-mediated signaling pathways. Thus, GSH is a promising target to protect brain endothelial cells in conditions of brain injury and disease.
Keywords: Reactive oxygen species (ROS); apoptosis; glutathione (GSH); hydrogen peroxide (H2O2); murine brain endothelial cells (bEnd.3 cells); nuclear factor erythroid 2-related factor 2 (Nrf2).
Figures
Similar articles
-
Exendin-4 Protects Human Retinal Pigment Epithelial Cells from H2O2-Induced Oxidative Damage via Activation of NRF2 Signaling.Ophthalmic Res. 2020;63(4):404-412. doi: 10.1159/000504891. Epub 2019 Dec 20. Ophthalmic Res. 2020. PMID: 31865348
-
Nuclear factor erythroid 2-related factor 2 antioxidant response element pathways protect bovine mammary epithelial cells against H2O2-induced oxidative damage in vitro.J Dairy Sci. 2018 Jun;101(6):5329-5344. doi: 10.3168/jds.2017-14128. Epub 2018 Mar 21. J Dairy Sci. 2018. PMID: 29573798
-
Protective effects of glutathione on oxidative injury induced by hydrogen peroxide in intestinal epithelial cells.J Surg Res. 2018 Feb;222:39-47. doi: 10.1016/j.jss.2017.09.041. Epub 2017 Oct 31. J Surg Res. 2018. PMID: 29273374
-
The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress.Free Radic Biol Med. 2013 Dec;65:1174-1194. doi: 10.1016/j.freeradbiomed.2013.09.001. Epub 2013 Sep 12. Free Radic Biol Med. 2013. PMID: 24036104 Review.
-
Exploring the multifaceted role of NRF2 in brain physiology and cancer: A comprehensive review.Neurooncol Adv. 2023 Dec 23;6(1):vdad160. doi: 10.1093/noajnl/vdad160. eCollection 2024 Jan-Dec. Neurooncol Adv. 2023. PMID: 38221979 Free PMC article. Review.
Cited by
-
Comprehensive assessment of genetic sequence variants in the antioxidant 'master regulator' NRF2 in idiopathic Parkinson's disease.PLoS One. 2015 May 26;10(5):e0128030. doi: 10.1371/journal.pone.0128030. eCollection 2015. PLoS One. 2015. PMID: 26010367 Free PMC article.
-
Ex-Vivo 13C NMR Spectroscopy of Rodent Brain: TNF Restricts Neuronal Utilization of Astrocyte-Derived Metabolites.J Proteome Res. 2024 Aug 2;23(8):3383-3392. doi: 10.1021/acs.jproteome.4c00035. Epub 2024 Jun 29. J Proteome Res. 2024. PMID: 38943617 Free PMC article.
-
ROS signaling and redox biology in endothelial cells.Cell Mol Life Sci. 2015 Sep;72(17):3281-303. doi: 10.1007/s00018-015-1928-9. Epub 2015 May 14. Cell Mol Life Sci. 2015. PMID: 25972278 Free PMC article. Review.
-
Self-Regulation of Cerebral Metabolism and Its Neuroprotective Effect After Hypoxic-Ischemic Injury: Evidence From 1H-MRS.Front Neuroanat. 2021 Jun 17;15:672412. doi: 10.3389/fnana.2021.672412. eCollection 2021. Front Neuroanat. 2021. PMID: 34220456 Free PMC article.
-
Data on metabolic-dependent antioxidant response in the cardiovascular tissues of living zebrafish under stress conditions.Data Brief. 2017 Apr 27;12:427-432. doi: 10.1016/j.dib.2017.04.034. eCollection 2017 Jun. Data Brief. 2017. PMID: 28516138 Free PMC article.
References
-
- Reliene R, Schiestl RH. Glutathione depletion by buthionine sulfoximine induces DNA deletions in mice. Carcinogenesis. 2006;27:240–244. - PubMed
-
- Anderson ME. Glutathione: an overview of biosynthesis and modulation. Chem Biol Interact. 1998;111-112:1–14. - PubMed
-
- Wu WC, Bhavsar JH, Aziz GF, Sadaniantz A. An overview of stress echocardiography in the study of patients with dilated or hypertrophic cardiomyopathy. Echocardiography. 2004;21:467–475. - PubMed
-
- Patten DA, Germain M, Kelly MA, Slack RS. Reactive oxygen species: stuck in the middle of neurodegeneration. J Alzheimers Dis. 2010;20(Suppl 2):S357–S367. - PubMed
-
- Nanetti L, Raffaelli F, Vignini A, Perozzi C, Silvestrini M, Bartolini M, Provinciali L, Mazzanti L. Oxidative stress in ischaemic stroke. Eur J Clin Invest. 2011;41:1318–1322. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

