Effects of combination antiretroviral therapies on the risk of myocardial infarction among HIV patients
- PMID: 24713880
- PMCID: PMC4159700
- DOI: 10.1097/EDE.0000000000000041
Effects of combination antiretroviral therapies on the risk of myocardial infarction among HIV patients
Abstract
Background: Cohort studies have demonstrated greater risk of myocardial infarction (MI) associated with specific antiretroviral use, while meta-analyses of randomized controlled trials (RCTs) have not. These differences may be due to inherent biases in the observational study design or to the limited duration of randomized trials. We conducted a new-user, active-comparator cohort study emulating an RCT comparing the initiation of several antiretrovirals as part of combination antiretroviral therapy (cART) and MI.
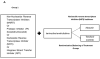
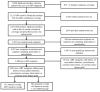
Methods: We included North Carolina (NC) Medicaid beneficiaries infected with human immunodeficiency virus between 2002 and 2008 who were previously untreated with cART. We compared hazard ratios (HRs) and 95% confidence intervals (CIs) of MI between abacavir and tenofovir recipients, and lopinavir-ritonavir or atazanavir recipients and nonnucleoside reverse transcriptase inhibitor (NNRTI) recipients. We adjusted for confounding through inverse probability weighting methods.
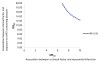
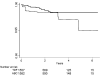
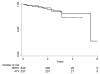
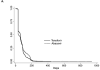
Results: There were 3481 NC Medicaid new cART recipients who contributed 6399 person-years and experienced 38 MI events. Receiving abacavir compared with tenofovir as part of cART was associated with an increased rate of MI (unadjusted HR = 2.70 [95% CI = 1.24-5.91]; adjusted HR = 2.05 [0.72-5.86]). Point estimates also suggest a relationship between receipt of atazanavir or lopinavir-ritonavir compared with an NNRTI and MI, although estimates were imprecise.
Conclusions: We found an increased rate of MI among patients initiating abacavir compared with tenofovir, although the association was decreased after confounding adjustment. Without a very large prospective comparative clinical trial, a much larger observational study of patients initiating cART would be needed to better define this apparent association.
Figures




Similar articles
-
Abacavir and risk of myocardial infarction in HIV-infected patients on highly active antiretroviral therapy: a population-based nationwide cohort study.HIV Med. 2010 Feb;11(2):130-6. doi: 10.1111/j.1468-1293.2009.00751.x. Epub 2009 Aug 13. HIV Med. 2010. PMID: 19682101
-
No risk of myocardial infarction associated with initial antiretroviral treatment containing abacavir: short and long-term results from ACTG A5001/ALLRT.Clin Infect Dis. 2011 Apr 1;52(7):929-40. doi: 10.1093/cid/ciq244. Clin Infect Dis. 2011. PMID: 21427402 Free PMC article. Clinical Trial.
-
Impact of individual antiretroviral drugs on the risk of myocardial infarction in human immunodeficiency virus-infected patients: a case-control study nested within the French Hospital Database on HIV ANRS cohort CO4.Arch Intern Med. 2010 Jul 26;170(14):1228-38. doi: 10.1001/archinternmed.2010.197. Arch Intern Med. 2010. PMID: 20660842
-
Co-formulated abacavir-lamivudine-zidovudine for initial treatment of HIV infection and AIDS.Cochrane Database Syst Rev. 2013 Mar 28;2013(3):CD005481. doi: 10.1002/14651858.CD005481.pub3. Cochrane Database Syst Rev. 2013. PMID: 23543540 Free PMC article. Review.
-
Initiation of antiretroviral therapy: implications of recent findings.Top HIV Med. 2004 Jul-Aug;12(3):83-8. Top HIV Med. 2004. PMID: 15310939 Review.
Cited by
-
Spirulina platensis Ameliorates Oxidative Stress Associated with Antiretroviral Drugs in HepG2 Cells.Plants (Basel). 2022 Nov 17;11(22):3143. doi: 10.3390/plants11223143. Plants (Basel). 2022. PMID: 36432871 Free PMC article.
-
Risk of cardiovascular events from current, recent, and cumulative exposure to abacavir among persons living with HIV who were receiving antiretroviral therapy in the United States: a cohort study.BMC Infect Dis. 2017 Oct 27;17(1):708. doi: 10.1186/s12879-017-2808-8. BMC Infect Dis. 2017. PMID: 29078761 Free PMC article.
-
Factors Associated With Excess Myocardial Infarction Risk in HIV-Infected Adults: A Systematic Review and Meta-analysis.J Acquir Immune Defic Syndr. 2019 Jun 1;81(2):224-230. doi: 10.1097/QAI.0000000000001996. J Acquir Immune Defic Syndr. 2019. PMID: 30865179 Free PMC article.
-
Lipid accumulation product index in HIV-infected patients: a marker of cardiovascular risk.Braz J Infect Dis. 2018 May-Jun;22(3):171-176. doi: 10.1016/j.bjid.2018.03.006. Epub 2018 Apr 22. Braz J Infect Dis. 2018. PMID: 29684319 Free PMC article.
-
HIV infection as vascular risk: A systematic review of the literature and meta-analysis.PLoS One. 2017 May 11;12(5):e0176686. doi: 10.1371/journal.pone.0176686. eCollection 2017. PLoS One. 2017. PMID: 28493892 Free PMC article. Review.
References
-
- Palella FJ, Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006 Sep;43(1):27–34. - PubMed
-
- Durand M, Sheehy O, Baril JG, et al. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: a cohort and nested case-control study using Quebec's public health insurance database. J Acquir Immune Defic Syndr. 2011 Jul 1;57(3):245–253. - PubMed
-
- Obel N, Farkas DK, Kronborg G, et al. Abacavir and risk of myocardial infarction in HIV-infected patients on highly active antiretroviral therapy: a population-based nationwide cohort study. HIV medicine. 2010 Feb;11(2):130–136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

