Protection against collagen-induced arthritis in mice afforded by the parasitic worm product, ES-62, is associated with restoration of the levels of interleukin-10-producing B cells and reduced plasma cell infiltration of the joints
- PMID: 24708419
- PMCID: PMC3930382
- DOI: 10.1111/imm.12208
Protection against collagen-induced arthritis in mice afforded by the parasitic worm product, ES-62, is associated with restoration of the levels of interleukin-10-producing B cells and reduced plasma cell infiltration of the joints
Abstract
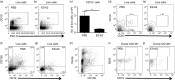
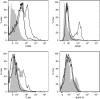
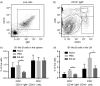
We have previously reported that ES-62, a molecule secreted by the parasitic filarial nematode Acanthocheilonema viteae, protects mice from developing collagen-induced arthritis (CIA). Together with increasing evidence that worm infection may protect against autoimmune conditions, this raises the possibility that ES-62 may have therapeutic potential in rheumatoid arthritis and hence, it is important to fully understand its mechanism of action. To this end, we have established to date that ES-62 protection in CIA is associated with suppressed T helper type 1 (Th1)/Th17 responses, reduced collagen-specific IgG2a antibodies and increased interleukin-10 (IL-10) production by splenocytes. IL-10-producing regulatory B cells have been proposed to suppress pathogenic Th1/Th17 responses in CIA: interestingly therefore, although the levels of IL-10-producing B cells were decreased in the spleens of mice with CIA, ES-62 was found to restore these to the levels found in naive mice. In addition, exposure to ES-62 decreased effector B-cell, particularly plasma cell, infiltration of the joints, and such infiltrating B cells showed dramatically reduced levels of Toll-like receptor 4 and the activation markers, CD80 and CD86. Collectively, this induction of hyporesponsiveness of effector B-cell responses, in the context of the resetting of the levels of IL-10-producing B cells, is suggestive of a modulation of the balance between effector and regulatory B-cell responses that may contribute to ES-62-mediated suppression of CIA-associated inflammation and inhibition of production of pathogenic collagen-specific IgG2a antibodies.
Keywords: ES-62; interleukin-10-producing B cells; parasitic helminths; rheumatoid arthritis.
© 2013 The Authors. Immunology published by John Wiley & Sons Ltd.
Figures



Similar articles
-
The parasitic helminth product ES-62 suppresses pathogenesis in collagen-induced arthritis by targeting the interleukin-17-producing cellular network at multiple sites.Arthritis Rheum. 2012 Oct;64(10):3168-78. doi: 10.1002/art.34581. Arthritis Rheum. 2012. PMID: 22729944
-
ES-62 protects against collagen-induced arthritis by resetting interleukin-22 toward resolution of inflammation in the joints.Arthritis Rheumatol. 2014 Jun;66(6):1492-503. doi: 10.1002/art.38392. Arthritis Rheumatol. 2014. PMID: 24497523 Free PMC article.
-
Suppression of inflammatory arthritis by the parasitic worm product ES-62 is associated with epigenetic changes in synovial fibroblasts.PLoS Pathog. 2021 Nov 8;17(11):e1010069. doi: 10.1371/journal.ppat.1010069. eCollection 2021 Nov. PLoS Pathog. 2021. PMID: 34748611 Free PMC article.
-
From the worm to the pill, the parasitic worm product ES-62 raises new horizons in the treatment of rheumatoid arthritis.Lupus. 2015 Apr;24(4-5):400-11. doi: 10.1177/0961203314560004. Lupus. 2015. PMID: 25801883 Review.
-
Lessons from helminth infections: ES-62 highlights new interventional approaches in rheumatoid arthritis.Clin Exp Immunol. 2014 Jul;177(1):13-23. doi: 10.1111/cei.12252. Clin Exp Immunol. 2014. PMID: 24666108 Free PMC article. Review.
Cited by
-
Particularities of allergy in the Tropics.World Allergy Organ J. 2016 Jun 27;9:20. doi: 10.1186/s40413-016-0110-7. eCollection 2016. World Allergy Organ J. 2016. PMID: 27386040 Free PMC article. Review.
-
Failure of the Anti-Inflammatory Parasitic Worm Product ES-62 to Provide Protection in Mouse Models of Type I Diabetes, Multiple Sclerosis, and Inflammatory Bowel Disease.Molecules. 2018 Oct 17;23(10):2669. doi: 10.3390/molecules23102669. Molecules. 2018. PMID: 30336585 Free PMC article.
-
Metabolic and adaptive immune responses induced in mice infected with tissue-dwelling nematode Trichinella zimbabwensis.Open Vet J. 2016;6(3):178-184. doi: 10.4314/ovj.v6i3.6. Epub 2016 Nov 4. Open Vet J. 2016. PMID: 27882304 Free PMC article.
-
The parasitic worm product ES-62 targets myeloid differentiation factor 88-dependent effector mechanisms to suppress antinuclear antibody production and proteinuria in MRL/lpr mice.Arthritis Rheumatol. 2015 Apr;67(4):1023-35. doi: 10.1002/art.39004. Arthritis Rheumatol. 2015. PMID: 25546822 Free PMC article.
-
Testing small molecule analogues of the Acanthocheilonema viteae immunomodulator ES-62 against clinically relevant allergens.Parasite Immunol. 2016 Jun;38(6):340-51. doi: 10.1111/pim.12322. Parasite Immunol. 2016. PMID: 27059010 Free PMC article.
References
-
- Panda AK, Ravindran B, Das BK. Rheumatoid arthritis patients are free of filarial infection in an area where filariasis is endemic: comment on the article by Pineda et al. Arthritis Rheum. 2013;65:1402–3. - PubMed
-
- Harnett W, Harnett MM. Reply: comment on Rheumatoid arthritis patients are free of filarial infection in an area where filariasis is endemic: comment on the article by Pineda et al [Arthritis Rheum. 2013] The parasitic helminth product ES-62 suppresses pathogenesis in collagen-induced arthritis by targeting the interleukin-17-producing cellular network at multiple sites. [Arthritis Rheum. 2012] Arthritis Rheum. 2013;65:1403–4. - PubMed
-
- Harnett W, Harnett MM. Helminth-derived immunomodulators: can understanding the worm produce the pill? Nat Rev Immunol. 2010;10:278–84. - PubMed
-
- Pineda MA, McGrath MA, Smith PC, Al-Riyami L, Rzepecka J, Gracie JA, Harnett W, Harnett MM. The parasitic helminth product ES-62 suppresses pathogenesis in CIA by targeting of the IL-17-producing cellular network at multiple sites. Arthritis Rheum. 2012;64:3168–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

