Fermented dairy products modulate Citrobacter rodentium-induced colonic hyperplasia
- PMID: 24706936
- PMCID: PMC4157696
- DOI: 10.1093/infdis/jiu205
Fermented dairy products modulate Citrobacter rodentium-induced colonic hyperplasia
Abstract
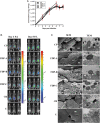
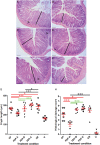
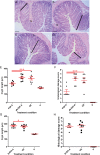
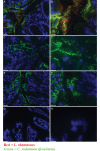
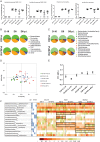
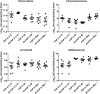
We evaluated the protective effects of fermented dairy products (FDPs) in an infection model, using the mouse pathogen Citrobacter rodentium (CR). Treatment of mice with FDP formulas A, B, and C or a control product did not affect CR colonization, organ specificity, or attaching and effacing lesion formation. Fermented dairy product A (FDP-A), but neither the supernatant from FDP-A nor β-irradiated (IR) FDP-A, caused a significant reduction in colonic crypt hyperplasia and CR-associated pathology. Profiling the gut microbiota revealed that IR-FDP-A promoted higher levels of phylotypes belonging to Alcaligenaceae and a decrease in Lachnospiraceae (Ruminococcus) during CR infection. Conversely, FDP-A prevented a decrease in Ruminococcus and increased Turicibacteraceae (Turicibacter). Importantly, loss of Ruminococcus and Turicibacter has been associated with susceptibility to dextran sodium sulfate-induced colitis. Our results demonstrate that viable bacteria in FDP-A reduced CR-induced colonic crypt hyperplasia and prevented the loss of key bacterial genera that may contribute to disease pathology.
Keywords: C. rodentium; DLIT-µCT; bioluminescence imaging; fermented dairy products; microbiota; probiotic.
© The Author 2014. Published by Oxford University Press on behalf of the Infectious Diseases Society of America.
Figures
Similar articles
-
Pre-treatment with Bifidobacterium breve UCC2003 modulates Citrobacter rodentium-induced colonic inflammation and organ specificity.Microbiology (Reading). 2012 Nov;158(Pt 11):2826-2834. doi: 10.1099/mic.0.060830-0. Epub 2012 Aug 17. Microbiology (Reading). 2012. PMID: 22902730 Free PMC article.
-
Osteopontin mediates Citrobacter rodentium-induced colonic epithelial cell hyperplasia and attaching-effacing lesions.Am J Pathol. 2010 Sep;177(3):1320-32. doi: 10.2353/ajpath.2010.091068. Epub 2010 Jul 22. Am J Pathol. 2010. PMID: 20651246 Free PMC article.
-
Preinoculation with the probiotic Lactobacillus acidophilus early in life effectively inhibits murine Citrobacter rodentium colitis.Pediatr Res. 2005 Dec;58(6):1185-91. doi: 10.1203/01.pdr.0000183660.39116.83. Pediatr Res. 2005. PMID: 16306191
-
Molecular pathogenesis of Citrobacter rodentium and transmissible murine colonic hyperplasia.Microbes Infect. 2001 Apr;3(4):333-40. doi: 10.1016/s1286-4579(01)01387-9. Microbes Infect. 2001. PMID: 11334751 Review.
-
Citrobacter rodentium of mice and man.Cell Microbiol. 2005 Dec;7(12):1697-706. doi: 10.1111/j.1462-5822.2005.00625.x. Cell Microbiol. 2005. PMID: 16309456 Review.
Cited by
-
Citrobacter rodentium mouse model of bacterial infection.Nat Protoc. 2016 Oct;11(10):1851-76. doi: 10.1038/nprot.2016.100. Epub 2016 Sep 8. Nat Protoc. 2016. PMID: 27606775
-
In vitro Study of Lactobacillus paracasei CNCM I-1518 in Healthy and Clostridioides difficile Colonized Elderly Gut Microbiota.Front Nutr. 2019 Dec 10;6:184. doi: 10.3389/fnut.2019.00184. eCollection 2019. Front Nutr. 2019. PMID: 31921877 Free PMC article.
-
Lactobacillus acidophilus counteracts inhibition of NHE3 and DRA expression and alleviates diarrheal phenotype in mice infected with Citrobacter rodentium.Am J Physiol Gastrointest Liver Physiol. 2016 Nov 1;311(5):G817-G826. doi: 10.1152/ajpgi.00173.2016. Epub 2016 Sep 15. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 27634011 Free PMC article.
-
Circadian Rhythm Perturbation Aggravates Gut Microbiota Dysbiosis in Dextran Sulfate Sodium-Induced Colitis in Mice.Nutrients. 2024 Jan 12;16(2):247. doi: 10.3390/nu16020247. Nutrients. 2024. PMID: 38257139 Free PMC article.
-
Administration of Bifidobacterium bifidum CGMCC 15068 modulates gut microbiota and metabolome in azoxymethane (AOM)/dextran sulphate sodium (DSS)-induced colitis-associated colon cancer (CAC) in mice.Appl Microbiol Biotechnol. 2020 Jul;104(13):5915-5928. doi: 10.1007/s00253-020-10621-z. Epub 2020 May 4. Appl Microbiol Biotechnol. 2020. PMID: 32367312
References
-
- Huebner ES, Surawicz CM. Probiotics in the prevention and treatment of gastrointestinal infections. Gastroenterol Clin North Am. 2006;35:355–65. - PubMed
-
- United Nations Food and Agriculture Organization. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. http://www.who.int/foodsafety/publications/fsmanagement/en/probiotics.pdf . Accessed 16 June 2009.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

