Persistence of skin-resident memory T cells within an epidermal niche
- PMID: 24706879
- PMCID: PMC3986170
- DOI: 10.1073/pnas.1322292111
Persistence of skin-resident memory T cells within an epidermal niche
Abstract
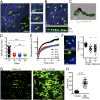
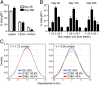
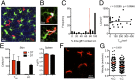
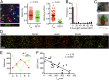
Barrier tissues such as the skin contain various populations of immune cells that contribute to protection from infections. These include recently identified tissue-resident memory T cells (TRM). In the skin, these memory CD8(+) T cells reside in the epidermis after being recruited to this site by infection or inflammation. In this study, we demonstrate prolonged persistence of epidermal TRM preferentially at the site of prior infection despite sustained migration. Computational simulation of TRM migration within the skin over long periods revealed that the slow rate of random migration effectively constrains these memory cells within the region of skin in which they form. Notably, formation of TRM involved a concomitant local reduction in dendritic epidermal γδ T-cell numbers in the epidermis, indicating that these populations persist in mutual exclusion and may compete for local survival signals. Accordingly, we show that expression of the aryl hydrocarbon receptor, a transcription factor important for dendritic epidermal γδ T-cell maintenance in skin, also contributes to the persistence of skin TRM. Together, these data suggest that skin tissue-resident memory T cells persist within a tightly regulated epidermal T-cell niche.
Keywords: Brownian motion; Langerhans cells; intravital imaging HSV-1 infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Niches for the Long-Term Maintenance of Tissue-Resident Memory T Cells.Front Immunol. 2018 May 31;9:1214. doi: 10.3389/fimmu.2018.01214. eCollection 2018. Front Immunol. 2018. PMID: 29904388 Free PMC article. Review.
-
Chemokine Receptor-Dependent Control of Skin Tissue-Resident Memory T Cell Formation.J Immunol. 2017 Oct 1;199(7):2451-2459. doi: 10.4049/jimmunol.1700571. Epub 2017 Aug 30. J Immunol. 2017. PMID: 28855310
-
Competition for Active TGFβ Cytokine Allows for Selective Retention of Antigen-Specific Tissue- Resident Memory T Cells in the Epidermal Niche.Immunity. 2021 Jan 12;54(1):84-98.e5. doi: 10.1016/j.immuni.2020.10.022. Epub 2020 Nov 18. Immunity. 2021. PMID: 33212014 Free PMC article.
-
CD4+ Resident Memory T Cells Mediate Long-Term Local Skin Immune Memory of Contact Hypersensitivity in BALB/c Mice.Front Immunol. 2020 May 19;11:775. doi: 10.3389/fimmu.2020.00775. eCollection 2020. Front Immunol. 2020. PMID: 32508808 Free PMC article.
-
Pathophysiology of Skin Resident Memory T Cells.Front Immunol. 2021 Feb 3;11:618897. doi: 10.3389/fimmu.2020.618897. eCollection 2020. Front Immunol. 2021. PMID: 33633737 Free PMC article. Review.
Cited by
-
(Not) Home alone: Antigen presenting cell - T Cell communication in barrier tissues.Front Immunol. 2022 Sep 29;13:984356. doi: 10.3389/fimmu.2022.984356. eCollection 2022. Front Immunol. 2022. PMID: 36248804 Free PMC article. Review.
-
Comparative analysis of interactions between aryl hydrocarbon receptor ligand binding domain with its ligands: a computational study.BMC Struct Biol. 2018 Dec 6;18(1):15. doi: 10.1186/s12900-018-0095-2. BMC Struct Biol. 2018. PMID: 30522477 Free PMC article.
-
Building vs. Rebuilding Epidermis: Comparison Embryonic Development and Adult Wound Repair.Front Cell Dev Biol. 2022 Jan 25;9:796080. doi: 10.3389/fcell.2021.796080. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35145968 Free PMC article. Review.
-
Unraveling the ECM-Immune Cell Crosstalk in Skin Diseases.Front Cell Dev Biol. 2019 May 7;7:68. doi: 10.3389/fcell.2019.00068. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31134198 Free PMC article. Review.
-
Tissue-resident T cell-derived cytokines eliminate herpes simplex virus-2-infected cells.J Clin Invest. 2020 Jun 1;130(6):2903-2919. doi: 10.1172/JCI132583. J Clin Invest. 2020. PMID: 32125285 Free PMC article.
References
-
- Heath WR, Carbone FR. The skin-resident and migratory immune system in steady state and memory: Innate lymphocytes, dendritic cells and T cells. Nat Immunol. 2013;14(10):978–985. - PubMed
-
- Gebhardt T, et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477(7363):216–219. - PubMed
-
- Gebhardt T, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10(5):524–530. - PubMed
-
- Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137–161. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

