TIM-1 glycoprotein binds the adhesion receptor P-selectin and mediates T cell trafficking during inflammation and autoimmunity
- PMID: 24703780
- PMCID: PMC4066214
- DOI: 10.1016/j.immuni.2014.03.004
TIM-1 glycoprotein binds the adhesion receptor P-selectin and mediates T cell trafficking during inflammation and autoimmunity
Abstract
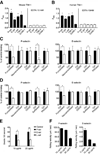
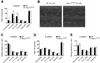
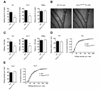
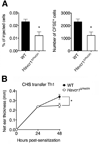
Selectins play a central role in leukocyte trafficking by mediating tethering and rolling on vascular surfaces. Here we have reported that T cell immunoglobulin and mucin domain 1 (TIM-1) is a P-selectin ligand. We have shown that human and murine TIM-1 binds to P-selectin, and that TIM-1 mediates tethering and rolling of T helper 1 (Th1) and Th17, but not Th2 and regulatory T cells on P-selectin. Th1 and Th17 cells lacking the TIM-1 mucin domain showed reduced rolling in thrombin-activated mesenteric venules and inflamed brain microcirculation. Inhibition of TIM-1 had no effect on naive T cell homing, but it reduced T cell recruitment in a skin hypersensitivity model and blocked experimental autoimmune encephalomyelitis. Uniquely, the TIM-1 immunoglobulin variable domain was also required for P-selectin binding. Our data demonstrate that TIM-1 is a major P-selectin ligand with a specialized role in T cell trafficking during inflammatory responses and the induction of autoimmune disease.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Efficient recruitment of lymphocytes in inflamed brain venules requires expression of cutaneous lymphocyte antigen and fucosyltransferase-VII.J Immunol. 2005 May 1;174(9):5805-13. doi: 10.4049/jimmunol.174.9.5805. J Immunol. 2005. PMID: 15843584
-
Regulation of T cell trafficking by the T cell immunoglobulin and mucin domain 1 glycoprotein.Trends Mol Med. 2014 Dec;20(12):675-84. doi: 10.1016/j.molmed.2014.10.003. Epub 2014 Oct 31. Trends Mol Med. 2014. PMID: 25457618 Review.
-
Host T cells are the main producers of IL-17 within the central nervous system during initiation of experimental autoimmune encephalomyelitis induced by adoptive transfer of Th1 cell lines.J Immunol. 2008 Jun 15;180(12):8066-72. doi: 10.4049/jimmunol.180.12.8066. J Immunol. 2008. PMID: 18523270
-
Overlapping roles of P-selectin and alpha 4 integrin to recruit leukocytes to the central nervous system in experimental autoimmune encephalomyelitis.J Immunol. 2002 Jul 15;169(2):1000-6. doi: 10.4049/jimmunol.169.2.1000. J Immunol. 2002. PMID: 12097407
-
The role of T cell immunoglobulin and mucin domain-3 in immune thrombocytopenia.Scand J Immunol. 2014 Apr;79(4):231-6. doi: 10.1111/sji.12153. Scand J Immunol. 2014. PMID: 24383985 Review.
Cited by
-
Distinct soluble immune checkpoint profiles characterize COVID-19 severity, mortality and SARS-CoV-2 variant infections.Front Immunol. 2024 Sep 23;15:1464480. doi: 10.3389/fimmu.2024.1464480. eCollection 2024. Front Immunol. 2024. PMID: 39376569 Free PMC article.
-
T Cell Response in Ischemic Stroke: From Mechanisms to Translational Insights.Front Immunol. 2021 Jul 15;12:707972. doi: 10.3389/fimmu.2021.707972. eCollection 2021. Front Immunol. 2021. PMID: 34335623 Free PMC article. Review.
-
The ability of human TIM1 to bind phosphatidylethanolamine enhances viral uptake and efferocytosis compared to rhesus and mouse orthologs.bioRxiv [Preprint]. 2024 Jul 29:2024.07.29.605603. doi: 10.1101/2024.07.29.605603. bioRxiv. 2024. Update in: J Virol. 2024 Nov 19;98(11):e0164924. doi: 10.1128/jvi.01649-24. PMID: 39131348 Free PMC article. Updated. Preprint.
-
Activating Natural Killer Cell Receptors, Selectins, and Inhibitory Siglecs Recognize Ebolavirus Glycoprotein.J Innate Immun. 2022;14(2):135-147. doi: 10.1159/000517628. Epub 2021 Aug 23. J Innate Immun. 2022. PMID: 34425576 Free PMC article.
-
Understanding T cell phenotype for the design of effective chimeric antigen receptor T cell therapies.J Immunother Cancer. 2021 May;9(5):e002555. doi: 10.1136/jitc-2021-002555. J Immunother Cancer. 2021. PMID: 34035114 Free PMC article. Review.
References
-
- Austrup F, Vestweber D, Borges E, Löhning M, Bräuer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature. 1997;385:81–83. - PubMed
-
- Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. - PubMed
-
- D’Ambrosio D, Lecca P, Constantin G, Priami C, Laudanna C. Concurrency in leukocyte vascular recognition: developing the tools for a predictive computer model. Trends Immunol. 2004;8:411–416. - PubMed
-
- Deban L, Russo RC, Sironi M, Moalli F, Scanziani M, Zambelli V, Cuccovillo I, Bastone A, Gobbi M, Valentino S, et al. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat. Immunol. 2010;11:328–334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

