Inhibition of KAP1 enhances hypoxia-induced Kaposi's sarcoma-associated herpesvirus reactivation through RBP-Jκ
- PMID: 24696491
- PMCID: PMC4054365
- DOI: 10.1128/JVI.00283-14
Inhibition of KAP1 enhances hypoxia-induced Kaposi's sarcoma-associated herpesvirus reactivation through RBP-Jκ
Abstract
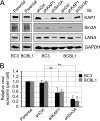
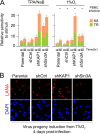
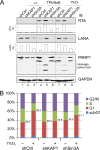
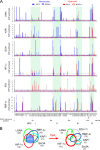
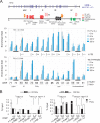
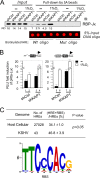
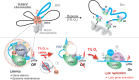
Hypoxia-inducible factor 1α (HIF-1α) has been frequently implicated in many cancers as well as viral pathogenesis. Kaposi's sarcoma-associated herpesvirus (KSHV) is linked to several human malignancies. It can stabilize HIF-1α during latent infection and undergoes lytic replication in response to hypoxic stress. However, the mechanism by which KSHV controls its latent and lytic life cycle through the deregulation of HIF-1α is not fully understood. Our previous studies showed that the hypoxia-sensitive chromatin remodeler KAP1 was targeted by the KSHV-encoded latency-associated nuclear antigen (LANA) to repress expression of the major lytic replication and transcriptional activator (RTA). Here we further report that an RNA interference-based knockdown of KAP1 in KSHV-infected primary effusion lymphoma (PEL) cells disrupted viral episome stability and abrogated sub-G1/G1 arrest of the cell cycle while increasing the efficiency of KSHV lytic reactivation by hypoxia or using the chemical 12-O-tetradecanoylphorbol-13-acetate (TPA) or sodium butyrate (NaB). Moreover, KSHV genome-wide screening revealed that four hypoxia-responsive clusters have a high concurrence of both RBP-Jκ and HIF-1α binding sites (RBS+HRE) within the same gene promoter and are tightly associated with KAP1. Inhibition of KAP1 greatly enhanced the association of RBP-Jκ with the HIF-1α complex for driving RTA expression not only in normoxia but also in hypoxia. These results suggest that both KAP1 and the concurrence of RBS+HRE within the RTA promoter are essential for KSHV latency and hypoxia-induced lytic reactivation.
Importance: Kaposi's sarcoma-associated herpesvirus (KSHV), a DNA tumor virus, is an etiological agent linked to several human malignancies, including Kaposi's sarcoma (KS) and primary effusion lymphoma (PEL). HIF-1α, a key hypoxia-inducible factor, is frequently elevated in KSHV latently infected tumor cells and contributes to KSHV lytic replication in hypoxia. The molecular mechanisms of how KSHV controls the latent and lytic life cycle through deregulating HIF-1α remain unclear. In this study, we found that inhibition of hypoxia-sensitive chromatin remodeler KAP1 in KSHV-infected PEL cells leads to a loss of viral genome and increases its sensitivity to hypoxic stress, leading to KSHV lytic reactivation. Importantly, we also found that four hypoxia-responsive clusters within the KSHV genome contain a high concurrence of RBP-Jκ (a key cellular regulator involved in Notch signaling) and HIF-1α binding sites. These sites are also tightly associated with KAP1. This discovery implies that KAP1, RBP-Jκ, and HIF-1α play an essential role in KSHV pathogenesis through subtle cross talk which is dependent on the oxygen levels in the infected cells.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Activated Nrf2 Interacts with Kaposi's Sarcoma-Associated Herpesvirus Latency Protein LANA-1 and Host Protein KAP1 To Mediate Global Lytic Gene Repression.J Virol. 2015 Aug;89(15):7874-92. doi: 10.1128/JVI.00895-15. Epub 2015 May 20. J Virol. 2015. PMID: 25995248 Free PMC article.
-
Genome-Wide Identification of Direct RTA Targets Reveals Key Host Factors for Kaposi's Sarcoma-Associated Herpesvirus Lytic Reactivation.J Virol. 2019 Feb 19;93(5):e01978-18. doi: 10.1128/JVI.01978-18. Print 2019 Mar 1. J Virol. 2019. PMID: 30541837 Free PMC article.
-
Effects of the NEDD8-Activating Enzyme Inhibitor MLN4924 on Lytic Reactivation of Kaposi's Sarcoma-Associated Herpesvirus.J Virol. 2017 Sep 12;91(19):e00505-17. doi: 10.1128/JVI.00505-17. Print 2017 Oct 1. J Virol. 2017. PMID: 28701396 Free PMC article.
-
Regulation of KSHV Latency and Lytic Reactivation.Viruses. 2020 Sep 17;12(9):1034. doi: 10.3390/v12091034. Viruses. 2020. PMID: 32957532 Free PMC article. Review.
-
Cyclooxygenase-2-prostaglandin E2-eicosanoid receptor inflammatory axis: a key player in Kaposi's sarcoma-associated herpes virus associated malignancies.Transl Res. 2013 Aug;162(2):77-92. doi: 10.1016/j.trsl.2013.03.004. Epub 2013 Apr 6. Transl Res. 2013. PMID: 23567332 Free PMC article. Review.
Cited by
-
KAP1 Positively Modulates Influenza A Virus Replication by Interacting with PB2 and NS1 Proteins in Human Lung Epithelial Cells.Viruses. 2022 Mar 26;14(4):689. doi: 10.3390/v14040689. Viruses. 2022. PMID: 35458419 Free PMC article.
-
A role of hypoxia-inducible factor 1 alpha in Murine Gammaherpesvirus 68 (MHV68) lytic replication and reactivation from latency.PLoS Pathog. 2019 Dec 6;15(12):e1008192. doi: 10.1371/journal.ppat.1008192. eCollection 2019 Dec. PLoS Pathog. 2019. PMID: 31809522 Free PMC article.
-
Merkel Cell Polyomavirus DNA Replication Induces Senescence in Human Dermal Fibroblasts in a Kap1/Trim28-Dependent Manner.mBio. 2020 Mar 10;11(2):e00142-20. doi: 10.1128/mBio.00142-20. mBio. 2020. PMID: 32156811 Free PMC article.
-
Nucleolar stress enhances lytic reactivation of the Kaposi's sarcoma-associated herpesvirus.Oncotarget. 2018 Feb 15;9(17):13822-13833. doi: 10.18632/oncotarget.24497. eCollection 2018 Mar 2. Oncotarget. 2018. PMID: 29568397 Free PMC article.
-
Role of Modulator of Inflammation Cyclooxygenase-2 in Gammaherpesvirus Mediated Tumorigenesis.Front Microbiol. 2017 Mar 28;8:538. doi: 10.3389/fmicb.2017.00538. eCollection 2017. Front Microbiol. 2017. PMID: 28400769 Free PMC article. Review.
References
-
- Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d'Agay MF, Clauvel JP, Raphael M, Degos L, Sigaux F. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276–1280 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

