Cysteine palmitoylation of the γ subunit has a dominant role in modulating activity of the epithelial sodium channel
- PMID: 24692558
- PMCID: PMC4022901
- DOI: 10.1074/jbc.M113.526020
Cysteine palmitoylation of the γ subunit has a dominant role in modulating activity of the epithelial sodium channel
Abstract
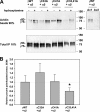
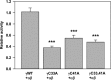
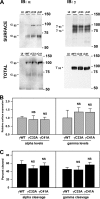
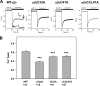
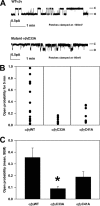
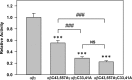
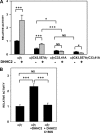
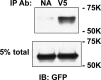
The epithelial sodium channel (ENaC) is composed of three homologous subunits (α, β, and γ) with cytoplasmic N and C termini. Our previous work revealed that two cytoplasmic Cys residues in the β subunit, βCys-43 and βCys-557, are Cys-palmitoylated. ENaCs with mutant βC43A/C557A exhibit normal surface expression but enhanced Na(+) self-inhibition and reduced channel open probability. Although the α subunit is not palmitoylated, we now show that the two cytoplasmic Cys residues in the γ subunit are palmitoylated. ENaCs with mutant γC33A, γC41A, or γC33A/C41A exhibit reduced activity compared with wild type channels but normal surface expression and normal levels of α and γ subunit-activating cleavage. These mutant channels have significantly enhanced Na(+) self-inhibition and reduced open probability compared with wild type ENaCs. Channel activity was enhanced by co-expression with the palmitoyltransferase DHHC2 that also co-immunoprecipitates with ENaCs. Secondary structure prediction of the N terminus of the γ subunit places γCys-33 within an α-helix and γCys-44 on a coil before the first transmembrane domain within a short tract that includes a well conserved His-Gly motif, where mutations have been associated with altered channel gating. Our current and previous results suggest that palmitoylation of the β and γ subunits of ENaCs enhances interactions of their respective cytoplasmic domains with the plasma membrane and stabilizes the open state of the channel. Comparison of activities of channels lacking palmitoylation sites in individual or multiple subunits revealed that γ subunit palmitoylation has a dominant role over β subunit palmitoylation in modulating ENaC gating.
Keywords: Acid-sensing Ion Channel (ASIC); ENaC; Gating; Ion Channels; Protein Palmitoylation.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
Similar articles
-
Specific Palmitoyltransferases Associate with and Activate the Epithelial Sodium Channel.J Biol Chem. 2017 Mar 10;292(10):4152-4163. doi: 10.1074/jbc.M117.776146. Epub 2017 Jan 30. J Biol Chem. 2017. PMID: 28154191 Free PMC article.
-
Cys palmitoylation of the beta subunit modulates gating of the epithelial sodium channel.J Biol Chem. 2010 Oct 1;285(40):30453-62. doi: 10.1074/jbc.M110.151845. Epub 2010 Jul 27. J Biol Chem. 2010. PMID: 20663869 Free PMC article.
-
Mice lacking γENaC palmitoylation sites maintain benzamil-sensitive Na+ transport despite reduced channel activity.JCI Insight. 2023 Nov 8;8(21):e172051. doi: 10.1172/jci.insight.172051. JCI Insight. 2023. PMID: 37707951 Free PMC article.
-
ASIC and ENaC type sodium channels: conformational states and the structures of the ion selectivity filters.FEBS J. 2017 Feb;284(4):525-545. doi: 10.1111/febs.13840. Epub 2016 Sep 15. FEBS J. 2017. PMID: 27580245 Review.
-
Regulating ENaC's gate.Am J Physiol Cell Physiol. 2020 Jan 1;318(1):C150-C162. doi: 10.1152/ajpcell.00418.2019. Epub 2019 Nov 13. Am J Physiol Cell Physiol. 2020. PMID: 31721612 Free PMC article. Review.
Cited by
-
Loss of the alpha subunit distal furin cleavage site blunts ENaC activation following Na+ restriction.J Physiol. 2024 Sep;602(17):4309-4326. doi: 10.1113/JP286559. Epub 2024 Aug 28. J Physiol. 2024. PMID: 39196791
-
The Epithelial Sodium Channel-An Underestimated Drug Target.Int J Mol Sci. 2023 Apr 24;24(9):7775. doi: 10.3390/ijms24097775. Int J Mol Sci. 2023. PMID: 37175488 Free PMC article. Review.
-
Palmitoylation of Voltage-Gated Ion Channels.Int J Mol Sci. 2022 Aug 19;23(16):9357. doi: 10.3390/ijms23169357. Int J Mol Sci. 2022. PMID: 36012639 Free PMC article. Review.
-
Epithelial Na + Channels Function as Extracellular Sensors.Compr Physiol. 2024 Mar 29;14(2):1-41. doi: 10.1002/cphy.c230015. Compr Physiol. 2024. PMID: 39109974 Review.
-
Force From Filaments: The Role of the Cytoskeleton and Extracellular Matrix in the Gating of Mechanosensitive Channels.Front Cell Dev Biol. 2022 May 2;10:886048. doi: 10.3389/fcell.2022.886048. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35586339 Free PMC article. Review.
References
-
- Satlin L. M., Carattino M. D., Liu W., Kleyman T. R. (2006) Regulation of cation transport in the distal nephron by mechanical forces. Am. J. Physiol. Renal Physiol. 291, F923–931 - PubMed
-
- Jasti J., Furukawa H., Gonzales E. B., Gouaux E. (2007) Structure of acid-sensing ion channel 1 at 1.9 Å resolution and low pH. Nature 449, 316–323 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

