Human astrocytes: secretome profiles of cytokines and chemokines
- PMID: 24691121
- PMCID: PMC3972155
- DOI: 10.1371/journal.pone.0092325
Human astrocytes: secretome profiles of cytokines and chemokines
Abstract
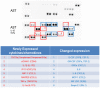
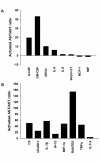
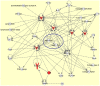
Astrocytes play a key role in maintenance of neuronal functions in the central nervous system by producing various cytokines, chemokines, and growth factors, which act as a molecular coordinator of neuron-glia communication. At the site of neuroinflammation, astrocyte-derived cytokines and chemokines play both neuroprotective and neurotoxic roles in brain lesions of human neurological diseases. At present, the comprehensive profile of human astrocyte-derived cytokines and chemokines during inflammation remains to be fully characterized. We investigated the cytokine secretome profile of highly purified human astrocytes by using a protein microarray. Non-stimulated human astrocytes in culture expressed eight cytokines, including G-CSF, GM-CSF, GROα (CXCL1), IL-6, IL-8 (CXCL8), MCP-1 (CCL2), MIF and Serpin E1. Following stimulation with IL-1β and TNF-α, activated astrocytes newly produced IL-1β, IL-1ra, TNF-α, IP-10 (CXCL10), MIP-1α (CCL3) and RANTES (CCL5), in addition to the induction of sICAM-1 and complement component 5. Database search indicated that most of cytokines and chemokines produced by non-stimulated and activated astrocytes are direct targets of the transcription factor NF-kB. These results indicated that cultured human astrocytes express a distinct set of NF-kB-target cytokines and chemokines in resting and activated conditions, suggesting that the NF-kB signaling pathway differentially regulates gene expression of cytokines and chemokines in human astrocytes under physiological and inflammatory conditions.
Conflict of interest statement
Figures

Similar articles
-
Regulation of beta-chemokine mRNA expression in adult rat astrocytes by lipopolysaccharide, proinflammatory and immunoregulatory cytokines.Scand J Immunol. 1998 Nov;48(5):502-8. doi: 10.1046/j.1365-3083.1998.00422.x. Scand J Immunol. 1998. PMID: 9822259
-
Negative feedback between prostaglandin and alpha- and beta-chemokine synthesis in human microglial cells and astrocytes.J Immunol. 1999 Feb 1;162(3):1701-6. J Immunol. 1999. PMID: 9973432
-
Cytokine regulation of CC and CXC chemokine expression by human astrocytes.J Neurovirol. 1999 Feb;5(1):82-94. doi: 10.3109/13550289909029749. J Neurovirol. 1999. PMID: 10190694
-
Potential neurotoxic activity of diverse molecules released by astrocytes.Brain Res Bull. 2022 Oct 15;189:80-101. doi: 10.1016/j.brainresbull.2022.08.015. Epub 2022 Aug 18. Brain Res Bull. 2022. PMID: 35988785 Review.
-
Roles of neuropathology-associated reactive astrocytes: a systematic review.Acta Neuropathol Commun. 2023 Mar 13;11(1):42. doi: 10.1186/s40478-023-01526-9. Acta Neuropathol Commun. 2023. PMID: 36915214 Free PMC article. Review.
Cited by
-
Whole-transcriptome brain expression and exon-usage profiling in major depression and suicide: evidence for altered glial, endothelial and ATPase activity.Mol Psychiatry. 2017 May;22(5):760-773. doi: 10.1038/mp.2016.130. Epub 2016 Aug 16. Mol Psychiatry. 2017. PMID: 27528462 Free PMC article.
-
Pharmacological ablation of astrocytes reduces Aβ degradation and synaptic connectivity in an ex vivo model of Alzheimer's disease.J Neuroinflammation. 2021 Mar 17;18(1):73. doi: 10.1186/s12974-021-02117-y. J Neuroinflammation. 2021. PMID: 33731156 Free PMC article.
-
Peripheral white blood cell patterns in children with hydrocephalus as a response to ventriculo-peritoneal shunt infection.PLoS One. 2024 Aug 9;19(8):e0308131. doi: 10.1371/journal.pone.0308131. eCollection 2024. PLoS One. 2024. PMID: 39121090 Free PMC article.
-
Autoantibodies in Neuropsychiatric Systemic Lupus Erythematosus (NPSLE): Can They Be Used as Biomarkers for the Differential Diagnosis of This Disease?Clin Rev Allergy Immunol. 2022 Oct;63(2):194-209. doi: 10.1007/s12016-021-08865-2. Epub 2021 Jun 11. Clin Rev Allergy Immunol. 2022. PMID: 34115263 Free PMC article. Review.
-
WIN 55,212-2, agonist of cannabinoid receptors, prevents amyloid β1-42 effects on astrocytes in primary culture.PLoS One. 2015 Apr 13;10(4):e0122843. doi: 10.1371/journal.pone.0122843. eCollection 2015. PLoS One. 2015. PMID: 25874692 Free PMC article.
References
-
- Barres BA (2008) The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60: 430–440. - PubMed
-
- Skalnikova H, Motlik J, Gadher SJ, Kovarova H (2011) Mapping of the secretome of primary isolates of mammalian cells, stem cells and derived cell lines. Proteomics 11: 691–708. - PubMed
-
- Kim SU, Moretto G, Lee V, Yu RK (1986) Neuroimmunology of gangliosides in human neurons and glial cells in culture. J Neurosci Res 15: 303–321. - PubMed
-
- Satoh J, Kim SU (1995) Cytokines and growth factors induce HSP27 phosphorylation in human astrocytes. J Neuropathol Exp Neurol 54: 504–512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

