Common drugs inhibit human organic cation transporter 1 (OCT1)-mediated neurotransmitter uptake
- PMID: 24688079
- PMCID: PMC4014663
- DOI: 10.1124/dmd.113.055095
Common drugs inhibit human organic cation transporter 1 (OCT1)-mediated neurotransmitter uptake
Abstract
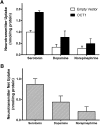
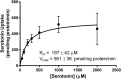
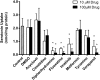
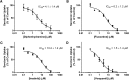
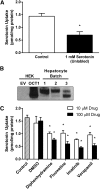
The human organic cation transporter 1 (OCT1) is a polyspecific transporter involved in the uptake of positively charged and neutral small molecules in the liver. To date, few endogenous compounds have been identified as OCT1 substrates; more importantly, the effect of drugs on endogenous substrate transport has not been examined. In this study, we established monoamine neurotransmitters as substrates for OCT1, specifically characterizing serotonin transport in human embryonic kidney 293 cells. Kinetic analysis yielded a Km of 197 micomolar and a Vmax of 561 pmol/mg protein/minute for serotonin. Furthermore, we demonstrated that serotonin uptake was inhibited by diphenhydramine, fluoxetine, imatinib, and verapamil, with IC50 values in the low micromolar range. These results were recapitulated in primary human hepatocytes, suggesting that OCT1 plays a significant role in hepatic elimination of serotonin and that xenobiotics may alter the elimination of endogenous compounds as a result of interactions at the transporter level.
Figures
Similar articles
-
Enhanced and Persistent Inhibition of Organic Cation Transporter 1 Activity by Preincubation of Cyclosporine A.Drug Metab Dispos. 2019 Nov;47(11):1352-1360. doi: 10.1124/dmd.119.087197. Epub 2019 Aug 19. Drug Metab Dispos. 2019. PMID: 31427432
-
Morphine is a substrate of the organic cation transporter OCT1 and polymorphisms in OCT1 gene affect morphine pharmacokinetics after codeine administration.Biochem Pharmacol. 2013 Sep 1;86(5):666-78. doi: 10.1016/j.bcp.2013.06.019. Epub 2013 Jul 5. Biochem Pharmacol. 2013. PMID: 23835420
-
Organic cation transporter 1 mediates the uptake of monocrotaline and plays an important role in its hepatotoxicity.Toxicology. 2013 Sep 15;311(3):225-30. doi: 10.1016/j.tox.2013.06.009. Epub 2013 Jul 3. Toxicology. 2013. PMID: 23831208
-
Pharmacological and physiological functions of the polyspecific organic cation transporters: OCT1, 2, and 3 (SLC22A1-3).J Pharmacol Exp Ther. 2004 Jan;308(1):2-9. doi: 10.1124/jpet.103.053298. Epub 2003 Oct 23. J Pharmacol Exp Ther. 2004. PMID: 14576340 Review.
-
Expression of organic cation transporter 1 (OCT1): unique patterns of indirect regulation by nuclear receptors and hepatospecific gene regulation.Drug Metab Rev. 2016 May;48(2):139-58. doi: 10.1080/03602532.2016.1188936. Epub 2016 Jun 9. Drug Metab Rev. 2016. PMID: 27278216 Review.
Cited by
-
General Overview of Organic Cation Transporters in Brain.Handb Exp Pharmacol. 2021;266:1-39. doi: 10.1007/164_2021_449. Handb Exp Pharmacol. 2021. PMID: 33782773
-
Development and Evaluation of an 18F-Radiolabeled Monocyclam Derivative for Imaging CXCR4 Expression.Mol Pharm. 2019 May 6;16(5):2106-2117. doi: 10.1021/acs.molpharmaceut.9b00069. Epub 2019 Apr 3. Mol Pharm. 2019. PMID: 30883140 Free PMC article.
-
Molecular basis of polyspecific drug and xenobiotic recognition by OCT1 and OCT2.Nat Struct Mol Biol. 2023 Jul;30(7):1001-1011. doi: 10.1038/s41594-023-01017-4. Epub 2023 Jun 8. Nat Struct Mol Biol. 2023. PMID: 37291422 Free PMC article.
-
Molecular basis of polyspecific drug binding and transport by OCT1 and OCT2.bioRxiv [Preprint]. 2023 Mar 16:2023.03.15.532610. doi: 10.1101/2023.03.15.532610. bioRxiv. 2023. Update in: Nat Struct Mol Biol. 2023 Jul;30(7):1001-1011. doi: 10.1038/s41594-023-01017-4. PMID: 36993738 Free PMC article. Updated. Preprint.
-
Heart insufficiency after combination of verapamil and metoprolol: A fatal case report and literature review.Clin Case Rep. 2019 Sep 19;7(11):2042-2048. doi: 10.1002/ccr3.2393. eCollection 2019 Nov. Clin Case Rep. 2019. PMID: 31788248 Free PMC article.
References
-
- Amphoux A, Vialou V, Drescher E, Brüss M, Mannoury La Cour C, Rochat C, Millan MJ, Giros B, Bönisch H, Gautron S. (2006) Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology 50:941–952 - PubMed
-
- Blackshear JL, Orlandi C, Hollenberg NK. (1986) Serotonin and the renal blood supply: role of prostaglandins and the 5HT-2 receptor. Kidney Int 30:304–310 - PubMed
-
- Blyden GT, Greenblatt DJ, Scavone JM, Shader RI. (1986) Pharmacokinetics of diphenhydramine and a demethylated metabolite following intravenous and oral administration. J Clin Pharmacol 26:529–533 - PubMed
-
- Bottlender R, Dobmeier P, Möller HJ. (1998) [The effect of selective serotonin-reuptake inhibitors in blood coagulation]. Fortschr Neurol Psychiatr 66:32–35 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

