Arginine vasopressin neuronal loss results from autophagy-associated cell death in a mouse model for familial neurohypophysial diabetes insipidus
- PMID: 24675466
- PMCID: PMC3973212
- DOI: 10.1038/cddis.2014.124
Arginine vasopressin neuronal loss results from autophagy-associated cell death in a mouse model for familial neurohypophysial diabetes insipidus
Abstract
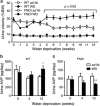
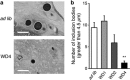
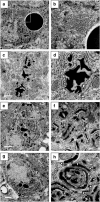
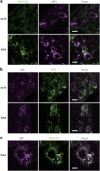
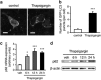
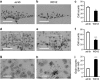
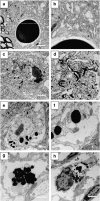
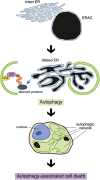
Familial neurohypophysial diabetes insipidus (FNDI) characterized by progressive polyuria is mostly caused by mutations in the gene encoding neurophysin II (NPII), which is the carrier protein of the antidiuretic hormone, arginine vasopressin (AVP). Although accumulation of mutant NPII in the endoplasmic reticulum (ER) could be toxic for AVP neurons, the precise mechanisms of cell death of AVP neurons, reported in autopsy studies, remain unclear. Here, we subjected FNDI model mice to intermittent water deprivation (WD) in order to promote the phenotypes. Electron microscopic analyses demonstrated that, while aggregates are confined to a certain compartment of the ER in the AVP neurons of FNDI mice with water access ad libitum, they were scattered throughout the dilated ER lumen in the FNDI mice subjected to WD for 4 weeks. It is also demonstrated that phagophores, the autophagosome precursors, emerged in the vicinity of aggregates and engulfed the ER containing scattered aggregates. Immunohistochemical analyses revealed that expression of p62, an adapter protein between ubiquitin and autophagosome, was elicited on autophagosomal membranes in the AVP neurons, suggesting selective autophagy induction at this time point. Treatment of hypothalamic explants of green fluorescent protein (GFP)-microtubule-associated protein 1 light chain 3 (LC3) transgenic mice with an ER stressor thapsigargin increased the number of GFP-LC3 puncta, suggesting that ER stress could induce autophagosome formation in the hypothalamus of wild-type mice as well. The cytoplasm of AVP neurons in FNDI mice was occupied with vacuoles in the mice subjected to WD for 12 weeks, when 30-40% of AVP neurons are lost. Our data thus demonstrated that autophagy was induced in the AVP neurons subjected to ER stress in FNDI mice. Although autophagy should primarily be protective for neurons, it is suggested that the organelles including ER were lost over time through autophagy, leading to autophagy-associated cell death of AVP neurons.
Figures
Similar articles
-
A novel mechanism of autophagy-associated cell death of vasopressin neurons in familial neurohypophysial diabetes insipidus.Cell Tissue Res. 2019 Jan;375(1):259-266. doi: 10.1007/s00441-018-2872-4. Epub 2018 Jun 30. Cell Tissue Res. 2019. PMID: 29961215 Review.
-
Progressive polyuria without vasopressin neuron loss in a mouse model for familial neurohypophysial diabetes insipidus.Am J Physiol Regul Integr Comp Physiol. 2009 May;296(5):R1641-9. doi: 10.1152/ajpregu.00034.2009. Epub 2009 Mar 18. Am J Physiol Regul Integr Comp Physiol. 2009. PMID: 19297548
-
Endoplasmic reticulum stress in vasopressin neurons of familial diabetes insipidus model mice: aggregate formation and mRNA poly(A) tail shortening.Exp Physiol. 2014 Jan;99(1):66-71. doi: 10.1113/expphysiol.2013.072553. Epub 2013 Oct 11. Exp Physiol. 2014. PMID: 24121282
-
Chemical chaperone 4-phenylbutylate reduces mutant protein accumulation in the endoplasmic reticulum of arginine vasopressin neurons in a mouse model for familial neurohypophysial diabetes insipidus.Neurosci Lett. 2018 Aug 24;682:50-55. doi: 10.1016/j.neulet.2018.06.013. Epub 2018 Jun 7. Neurosci Lett. 2018. PMID: 29886132
-
Mechanisms underlying progressive polyuria in familial neurohypophysial diabetes insipidus.J Neuroendocrinol. 2010 Jul;22(7):754-7. doi: 10.1111/j.1365-2826.2010.02028.x. Epub 2010 May 13. J Neuroendocrinol. 2010. PMID: 20492364 Review.
Cited by
-
Endoplasmic reticulum chaperone BiP/GRP78 knockdown leads to autophagy and cell death of arginine vasopressin neurons in mice.Sci Rep. 2020 Nov 12;10(1):19730. doi: 10.1038/s41598-020-76839-z. Sci Rep. 2020. PMID: 33184425 Free PMC article.
-
Degradation of Mutant Protein Aggregates within the Endoplasmic Reticulum of Vasopressin Neurons.iScience. 2020 Oct 7;23(10):101648. doi: 10.1016/j.isci.2020.101648. eCollection 2020 Oct 23. iScience. 2020. PMID: 33103081 Free PMC article.
-
Arginine vasopressin and pathophysiology of COVID-19: An innovative perspective.Biomed Pharmacother. 2021 Nov;143:112193. doi: 10.1016/j.biopha.2021.112193. Epub 2021 Sep 15. Biomed Pharmacother. 2021. PMID: 34543987 Free PMC article. Review.
-
Differentiation of human induced pluripotent stem cells into hypothalamic vasopressin neurons with minimal exogenous signals and partial conversion to the naive state.Sci Rep. 2022 Oct 17;12(1):17381. doi: 10.1038/s41598-022-22405-8. Sci Rep. 2022. PMID: 36253431 Free PMC article.
-
Two novel mutations in seven Czech and Slovak kindreds with familial neurohypophyseal diabetes insipidus-benefit of genetic testing.Eur J Pediatr. 2016 Sep;175(9):1199-1207. doi: 10.1007/s00431-016-2759-x. Epub 2016 Aug 18. Eur J Pediatr. 2016. PMID: 27539621
References
-
- Robertson GL.Posterior pituitaryIn: Felig P, Baxter JD, Frohman LA (eds)Endocrinology and Metabolism McGraw-Hill: New York; 1995385–432.
-
- Christensen JH, Rittig S. Familial neurohypophyseal diabetes insipidus—an update. Semin Nephrol. 2006;26:209–223. - PubMed
-
- Babey M, Kopp P, Robertson GL. Familial forms of diabetes insipidus: clinical and molecular characteristics. Nat Rev Endocrinol. 2011;7:701–714. - PubMed
-
- Birk J, Friberg MA, Prescianotto-Baschong C, Spiess M, Rutishauser J. Dominant pro-vasopressin mutants that cause diabetes insipidus form disulfide-linked fibrillar aggregates in the endoplasmic reticulum. J Cell Sci. 2009;122:3994–4002. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

