The role of muscle microRNAs in repairing the neuromuscular junction
- PMID: 24664281
- PMCID: PMC3963997
- DOI: 10.1371/journal.pone.0093140
The role of muscle microRNAs in repairing the neuromuscular junction
Abstract
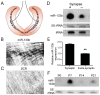
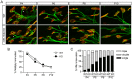
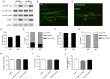
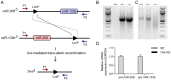
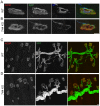
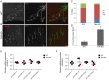
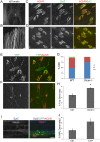
microRNAs have been implicated in mediating key aspects of skeletal muscle development and responses to diseases and injury. Recently, we demonstrated that a synaptically enriched microRNA, miR-206, functions to promote maintenance and repair of the neuromuscular junction (NMJ); in mutant mice lacking miR-206, reinnervation is impaired following nerve injury and loss of NMJs is accelerated in a mouse model of amyotrophic lateral sclerosis (ALS). Here, we asked whether other microRNAs play similar roles. One attractive candidate is miR-133b because it is in the same transcript that encodes miR-206. Like miR-206, miR-133b is concentrated near NMJs and induced after denervation. In miR-133b null mice, however, NMJ development is unaltered, reinnervation proceeds normally following nerve injury, and disease progression is unaffected in the SOD1(G93A) mouse model of ALS. To determine if miR-206 compensates for the loss of miR-133b, we generated mice lacking both microRNAs. The phenotype of these double mutants resembled that of miR-206 single mutants. Finally, we used conditional mutants of Dicer, an enzyme required for the maturation of most microRNAs, to generate mice in which microRNAs were depleted from skeletal muscle fibers postnatally, thus circumventing a requirement for microRNAs in embryonic muscle development. Reinnervation of muscle fibers following injury was impaired in these mice, but the defect was similar in magnitude to that observed in miR-206 mutants. Together, these results suggest that miR-206 is the major microRNA that regulates repair of the NMJ following nerve injury.
Conflict of interest statement
Figures
Similar articles
-
Argonaute 2 is lost from neuromuscular junctions affected with amyotrophic lateral sclerosis in SOD1G93A mice.Sci Rep. 2022 Mar 17;12(1):4630. doi: 10.1038/s41598-022-08455-y. Sci Rep. 2022. PMID: 35301367 Free PMC article.
-
Properties of Glial Cell at the Neuromuscular Junction Are Incompatible with Synaptic Repair in the SOD1G37R ALS Mouse Model.J Neurosci. 2020 Sep 30;40(40):7759-7777. doi: 10.1523/JNEUROSCI.1748-18.2020. Epub 2020 Aug 28. J Neurosci. 2020. PMID: 32859714 Free PMC article.
-
Muscle Fibers Secrete FGFBP1 to Slow Degeneration of Neuromuscular Synapses during Aging and Progression of ALS.J Neurosci. 2017 Jan 4;37(1):70-82. doi: 10.1523/JNEUROSCI.2992-16.2016. J Neurosci. 2017. PMID: 28053031 Free PMC article.
-
Therapeutics Targeting Skeletal Muscle in Amyotrophic Lateral Sclerosis.Biomolecules. 2024 Jul 22;14(7):878. doi: 10.3390/biom14070878. Biomolecules. 2024. PMID: 39062592 Free PMC article. Review.
-
Neuromuscular Junction as an Entity of Nerve-Muscle Communication.Cells. 2019 Aug 16;8(8):906. doi: 10.3390/cells8080906. Cells. 2019. PMID: 31426366 Free PMC article. Review.
Cited by
-
The role of microRNAs in skeletal muscle health and disease.Front Biosci (Landmark Ed). 2015 Jan 1;20(1):37-77. doi: 10.2741/4298. Front Biosci (Landmark Ed). 2015. PMID: 25553440 Free PMC article. Review.
-
The Cellular and Molecular Signature of ALS in Muscle.J Pers Med. 2022 Nov 8;12(11):1868. doi: 10.3390/jpm12111868. J Pers Med. 2022. PMID: 36579600 Free PMC article. Review.
-
Study on variation trend of repetitive nerve stimulation waveform in amyotrophic lateral sclerosis.Chin Med J (Engl). 2019 Mar 5;132(5):542-550. doi: 10.1097/CM9.0000000000000117. Chin Med J (Engl). 2019. PMID: 30807353 Free PMC article.
-
Failed reinnervation in aging skeletal muscle.Skelet Muscle. 2016 Sep 1;6(1):29. doi: 10.1186/s13395-016-0101-y. eCollection 2016. Skelet Muscle. 2016. PMID: 27588166 Free PMC article.
-
Transcriptomic Analysis of Zebrafish TDP-43 Transgenic Lines.Front Mol Neurosci. 2018 Dec 13;11:463. doi: 10.3389/fnmol.2018.00463. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30618614 Free PMC article.
References
-
- Sanes JR, Lichtman JW (1999) Development of the vertebrate neuromuscular junction. Annu Rev Neurosci 22: 389–442. - PubMed
-
- Balice-Gordon RJ (1997) Age-related changes in neuromuscular innervation. Muscle Nerve Suppl 5: S83–7. - PubMed
-
- Bruijn LI, Miller TM, Cleveland DW (2004) Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci 27: 723–749. - PubMed
-
- Punga AR, Ruegg MA (2012) Signaling and aging at the neuromuscular synapse: lessons learnt from neuromuscular diseases. Curr Opin Pharmacol 12: 340–346. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

