Prolonged administration of azacitidine with or without entinostat for myelodysplastic syndrome and acute myeloid leukemia with myelodysplasia-related changes: results of the US Leukemia Intergroup trial E1905
- PMID: 24663049
- PMCID: PMC3986386
- DOI: 10.1200/JCO.2013.50.3102
Prolonged administration of azacitidine with or without entinostat for myelodysplastic syndrome and acute myeloid leukemia with myelodysplasia-related changes: results of the US Leukemia Intergroup trial E1905
Abstract
Purpose: Although azacitidine (AZA) improves survival in patients with high-risk myelodysplastic syndrome, the overall response remains approximately 50%. Entinostat is a histone deacetylase inhibitor that has been combined with AZA with significant clinical activity in a previous phase I dose finding study.
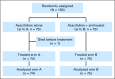
Design: Open label phase II randomized trial comparing AZA 50 mg/m(2)/d given for 10 days ± entinostat 4 mg/m(2)/d day 3 and day 10. All subtypes of myelodysplasia, chronic myelomonocytic leukemia, and acute myeloid leukemia with myelodysplasia-related changes were eligible for the study. The primary objective was the rate of hematologic normalization (HN; complete remission + partial remission + trilineage hematological improvement).
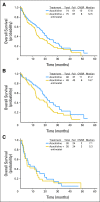
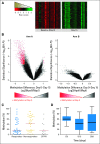
Results: One hundred forty-nine patients were analyzed, including 97 patients with myelodysplastic syndrome and 52 patients with acute myeloid leukemia. In the AZA group, 32% (95% CI, 22% to 44%) experienced HN and 27% (95% CI, 17% to 39%) in the AZA + entinostat group. Both arms exceeded the HN rate of historical control (Cancer and Leukemia Group B 9221 trial), but only the AZA group fulfilled the primary objective of the study. Rates of overall hematologic response were 46% and 44%, respectively. Median overall survivals were 18 months for the AZA group and 13 months for the AZA + entinostat group. The combination arm led to less demethylation compared with the monotherapy arm, suggesting pharmacodynamic antagonism.
Conclusion: Addition of entinostat to AZA did not increase clinical response as defined by the protocol and was associated with pharmacodynamic antagonism. However, the prolonged administration of AZA by itself seems to increase HN rate compared with standard dosing and warrants additional investigation.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Similar articles
-
Azacitidine with or without Entinostat for the treatment of therapy-related myeloid neoplasm: further results of the E1905 North American Leukemia Intergroup study.Br J Haematol. 2016 Feb;172(3):384-91. doi: 10.1111/bjh.13832. Epub 2015 Nov 18. Br J Haematol. 2016. PMID: 26577691 Free PMC article. Clinical Trial.
-
Feasibility of allogeneic stem-cell transplantation after azacitidine bridge in higher-risk myelodysplastic syndromes and low blast count acute myeloid leukemia: results of the BMT-AZA prospective study.Ann Oncol. 2017 Jul 1;28(7):1547-1553. doi: 10.1093/annonc/mdx154. Ann Oncol. 2017. PMID: 28368509 Clinical Trial.
-
Real Life Data on Efficacy and Safety of Azacitidine Therapy for Myelodysplastic Syndrome, Chronic Myelomonocytic Leukemia and Acute Myeloid Leukemia.Pathol Oncol Res. 2019 Jul;25(3):1175-1180. doi: 10.1007/s12253-018-00574-0. Epub 2019 Jan 6. Pathol Oncol Res. 2019. PMID: 30613922 Free PMC article.
-
Azacitidine for the treatment of myelodysplastic syndrome, chronic myelomonocytic leukaemia and acute myeloid leukaemia.Health Technol Assess. 2010 May;14 Suppl 1:69-74. doi: 10.3310/hta14Suppl1/10. Health Technol Assess. 2010. PMID: 20507806 Review.
-
[Azacitidine treatment for acute myeloid leukemia with myelodysplasia-related changes during peritoneal dialysis].Rinsho Ketsueki. 2017;58(12):2369-2374. doi: 10.11406/rinketsu.58.2369. Rinsho Ketsueki. 2017. PMID: 29332868 Review. Japanese.
Cited by
-
Investigational histone deacetylase inhibitors (HDACi) in myeloproliferative neoplasms.Expert Opin Investig Drugs. 2016 Dec;25(12):1393-1403. doi: 10.1080/13543784.2016.1250882. Epub 2016 Oct 31. Expert Opin Investig Drugs. 2016. PMID: 27756180 Free PMC article. Review.
-
Hypomethylating agent combination strategies in myelodysplastic syndromes: hopes and shortcomings.Leuk Lymphoma. 2017 May;58(5):1022-1036. doi: 10.1080/10428194.2016.1228927. Epub 2016 Sep 21. Leuk Lymphoma. 2017. PMID: 27654579 Free PMC article. Review.
-
Precision therapy for acute myeloid leukemia.J Hematol Oncol. 2018 Jan 5;11(1):3. doi: 10.1186/s13045-017-0543-7. J Hematol Oncol. 2018. PMID: 29301553 Free PMC article. Review.
-
More is less, less is more, or does it really matter? The curious case of impact of azacitidine administration schedules on outcomes in patients with myelodysplastic syndromes.BMC Hematol. 2018 Feb 1;18:4. doi: 10.1186/s12878-018-0095-2. eCollection 2018. BMC Hematol. 2018. PMID: 29435332 Free PMC article.
-
Beyond the Edge of Hypomethylating Agents: Novel Combination Strategies for Older Adults with Advanced MDS and AML.Cancers (Basel). 2018 May 24;10(6):158. doi: 10.3390/cancers10060158. Cancers (Basel). 2018. PMID: 29795051 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- U10 CA027057/CA/NCI NIH HHS/United States
- N01 CA032102/CA/NCI NIH HHS/United States
- R01 CA125563501/CA/NCI NIH HHS/United States
- R01 CA125635/CA/NCI NIH HHS/United States
- U10 CA037403/CA/NCI NIH HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- N01 CA027057/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA180794/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- K24 CA111717/CA/NCI NIH HHS/United States
- U24 CA114737/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

