Proresolving lipid mediators and mechanisms in the resolution of acute inflammation
- PMID: 24656045
- PMCID: PMC4004957
- DOI: 10.1016/j.immuni.2014.02.009
Proresolving lipid mediators and mechanisms in the resolution of acute inflammation
Abstract
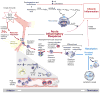
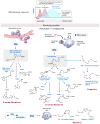
Inflammatory responses, like all biological cascades, are shaped by a delicate balance between positive and negative feedback loops. It is now clear that in addition to positive and negative checkpoints, the inflammatory cascade rather unexpectedly boasts an additional checkpoint, a family of chemicals that actively promote resolution and tissue repair without compromising host defense. Indeed, the resolution phase of inflammation is just as actively orchestrated and carefully choreographed as its induction and inhibition. In this review, we explore the immunological consequences of omega-3-derived specialized proresolving mediators (SPMs) and discuss their place within what is currently understood of the role of the arachidonic acid-derived prostaglandins, lipoxins, and their natural C15-epimers. We propose that treatment of inflammation should not be restricted to the use of inhibitors of the acute cascade (antagonism) but broadened to take account of the enormous therapeutic potential of inducers (agonists) of the resolution phase of inflammation.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Lipid mediators in the resolution of inflammation.Cold Spring Harb Perspect Biol. 2014 Oct 30;7(2):a016311. doi: 10.1101/cshperspect.a016311. Cold Spring Harb Perspect Biol. 2014. PMID: 25359497 Free PMC article. Review.
-
Maresins: Specialized Proresolving Lipid Mediators and Their Potential Role in Inflammatory-Related Diseases.Mediators Inflamm. 2018 Feb 20;2018:2380319. doi: 10.1155/2018/2380319. eCollection 2018. Mediators Inflamm. 2018. PMID: 29674943 Free PMC article. Review.
-
Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways.Annu Rev Immunol. 2007;25:101-37. doi: 10.1146/annurev.immunol.25.022106.141647. Annu Rev Immunol. 2007. PMID: 17090225 Review.
-
Anti-inflammatory and proresolving lipid mediators.Annu Rev Pathol. 2008;3:279-312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. Annu Rev Pathol. 2008. PMID: 18233953 Free PMC article. Review.
-
Specialized pro-resolving mediator network: an update on production and actions.Essays Biochem. 2020 Sep 23;64(3):443-462. doi: 10.1042/EBC20200018. Essays Biochem. 2020. PMID: 32885825 Free PMC article. Review.
Cited by
-
Dynamic monocyte chemoattractant protein-1 level as predictors of perceived pain during first and second phacoemulsification eye surgeries in patients with bilateral cataract.BMC Ophthalmol. 2021 Mar 12;21(1):133. doi: 10.1186/s12886-021-01880-z. BMC Ophthalmol. 2021. PMID: 33711968 Free PMC article.
-
Macrophage-Mediated Inflammation in Skin Wound Healing.Cells. 2022 Sep 21;11(19):2953. doi: 10.3390/cells11192953. Cells. 2022. PMID: 36230913 Free PMC article. Review.
-
Association of Single Nucleotide Polymorphisms (SNPs) of Chemoattractant Receptor23 (ChemR23) Gene with Susceptibility to Allergic Rhinitis.Biochem Genet. 2024 Aug;62(4):2587-2605. doi: 10.1007/s10528-023-10561-z. Epub 2023 Nov 22. Biochem Genet. 2024. PMID: 37993706
-
Human lung fibroblasts produce proresolving peroxisome proliferator-activated receptor-γ ligands in a cyclooxygenase-2-dependent manner.Am J Physiol Lung Cell Mol Physiol. 2016 Nov 1;311(5):L855-L867. doi: 10.1152/ajplung.00272.2016. Epub 2016 Sep 9. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 27612965 Free PMC article.
-
MerTK signaling in macrophages promotes the synthesis of inflammation resolution mediators by suppressing CaMKII activity.Sci Signal. 2018 Sep 25;11(549):eaar3721. doi: 10.1126/scisignal.aar3721. Sci Signal. 2018. PMID: 30254055 Free PMC article.
References
-
- Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol. 2004;173:559–565. - PubMed
-
- Aronoff DM, Carstens JK, Chen GH, Toews GB, Peters-Golden M. Short communication: differences between macrophages and dendritic cells in the cyclic AMP-dependent regulation of lipopolysaccharide-induced cytokine and chemokine synthesis. J Interferon Cytokine Res. 2006;26:827–833. - PubMed
-
- Aronoff DM, Peres CM, Serezani CH, Ballinger MN, Carstens JK, Coleman N, Moore BB, Peebles RS, Faccioli LH, Peters-Golden M. Synthetic prostacyclin analogs differentially regulate macrophage function via distinct analog-receptor binding specificities. J Immunol. 2007;178:1628–1634. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

