Synergistic effect of bone marrow-derived mesenchymal stem cells and platelet-rich plasma in streptozotocin-induced diabetic rats
- PMID: 24648680
- PMCID: PMC3956772
- DOI: 10.5021/ad.2014.26.1.1
Synergistic effect of bone marrow-derived mesenchymal stem cells and platelet-rich plasma in streptozotocin-induced diabetic rats
Abstract
Background: Diabetic wounds are a major clinical challenge, because minor skin wounds can lead to chronic, unhealed ulcers and ultimately result in infection, gangrene, or even amputation. Studies on bone marrow derived mesenchymal stem cells (BMSCs) and a series of growth factors have revealed their many benefits for wound healing and regeneration. Platelet-rich plasma (PRP) may improve the environment for BMSC development and differentiation. However, whether combined use of BMSCs and PRP may be more effective for accelerating diabetic ulcer healing remains unclear.
Objective: We investigated the efficacy of BMSCs and PRP for the repair of refractory wound healing in a diabetic rat model.
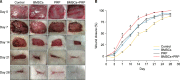
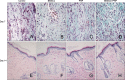
Methods: Forty-eight rats with diabetes mellitus induced by streptozotocin were divided into four groups: treatment with BMSCs plus PRP, BMSCs alone, PRP alone, phosphate buffered saline. The rate of wound closure was quantified. A histopathological study was conducted regarding wound depth and the skin edge at 7, 14, and 28 days after surgery.
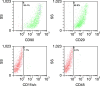
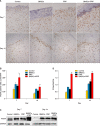
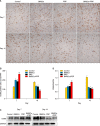
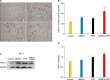
Results: Wound healing rates were significantly higher in the BMSC plus PRP group than in the other groups. The immunohistochemistry results showed that the expression of platelet/endothelial cell adhesion molecule 1, proliferating cell nuclear antigen, and transforming growth factor-β1 increased significantly in the BMSC plus PRP group compared to the other treatment groups. On day 7, CD68 expression increased significantly in the wounds of the BMSC plus PRP group, but decreased markedly at day 14 compared to the controls.
Conclusion: The combination of BMSCs and PRP aids diabetic wound repair and regeneration.
Keywords: Bone marrow-derived mesenchymal stem cell; Diabetes mellitus; Platelet-rich plasma; Wounds.
Figures

Similar articles
-
Combining mesenchymal stem cell sheets with platelet-rich plasma gel/calcium phosphate particles: a novel strategy to promote bone regeneration.Stem Cell Res Ther. 2015 Dec 21;6:256. doi: 10.1186/s13287-015-0256-1. Stem Cell Res Ther. 2015. PMID: 26689714 Free PMC article.
-
A novel kartogenin-platelet-rich plasma gel enhances chondrogenesis of bone marrow mesenchymal stem cells in vitro and promotes wounded meniscus healing in vivo.Stem Cell Res Ther. 2019 Jul 8;10(1):201. doi: 10.1186/s13287-019-1314-x. Stem Cell Res Ther. 2019. PMID: 31287023 Free PMC article.
-
[Effects of human adipose-derived mesenchymal stem cells and platelet-rich plasma on healing of wounds with full-thickness skin defects in mice].Zhonghua Shao Shang Za Zhi. 2018 Dec 20;34(12):887-894. doi: 10.3760/cma.j.issn.1009-2587.2018.12.013. Zhonghua Shao Shang Za Zhi. 2018. PMID: 30585053 Chinese.
-
The growing evidence for the use of platelet-rich plasma on diabetic chronic wounds: A review and a proposal for a new standard care.Wound Repair Regen. 2015 Sep;23(5):638-43. doi: 10.1111/wrr.12317. Epub 2015 Aug 25. Wound Repair Regen. 2015. PMID: 26019054 Review.
-
Autologous platelet-rich plasma for treating chronic wounds.Cochrane Database Syst Rev. 2012 Oct 17;10:CD006899. doi: 10.1002/14651858.CD006899.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2016 May 25;(5):CD006899. doi: 10.1002/14651858.CD006899.pub3. PMID: 23076929 Updated. Review.
Cited by
-
Effect of Bone Marrow Aspirate Concentrate-Platelet-Rich Plasma on Tendon-Derived Stem Cells and Rotator Cuff Tendon Tear.Cell Transplant. 2017 May 9;26(5):867-878. doi: 10.3727/096368917X694705. Epub 2017 Jan 20. Cell Transplant. 2017. PMID: 28105983 Free PMC article. Clinical Trial.
-
Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020.Int J Mol Sci. 2020 Oct 21;21(20):7794. doi: 10.3390/ijms21207794. Int J Mol Sci. 2020. PMID: 33096812 Free PMC article. Review.
-
In Vitro Evaluation of Proliferation and Migration Behaviour of Human Bone Marrow-Derived Mesenchymal Stem Cells in Presence of Platelet-Rich Plasma.Int J Dent. 2019 Apr 9;2019:9639820. doi: 10.1155/2019/9639820. eCollection 2019. Int J Dent. 2019. PMID: 31093287 Free PMC article.
-
Platelet Rich Plasma: New Insights for Cutaneous Wound Healing Management.J Funct Biomater. 2018 Jan 18;9(1):10. doi: 10.3390/jfb9010010. J Funct Biomater. 2018. PMID: 29346333 Free PMC article. Review.
-
Umbilical cord mesenchymal stem cells combined with autologous platelet-rich plasma for lower extremity venous ulcers: A case report and literature review.Medicine (Baltimore). 2024 Nov 8;103(45):e40433. doi: 10.1097/MD.0000000000040433. Medicine (Baltimore). 2024. PMID: 39533589 Free PMC article. Review.
References
-
- Blumberg SN, Berger A, Hwang L, Pastar I, Warren SM, Chen W. The role of stem cells in the treatment of diabetic foot ulcers. Diabetes Res Clin Pract. 2012;96:1–9. - PubMed
-
- Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75–81. - PubMed
-
- Ansurudeen I, Sunkari VG, Grünler J, Peters V, Schmitt CP, Catrina SB, et al. Carnosine enhances diabetic wound healing in the db/db mouse model of type 2 diabetes. Amino Acids. 2012;43:127–134. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

