Update on vaccine-derived polioviruses - worldwide, July 2012-December 2013
- PMID: 24647401
- PMCID: PMC4584635
Update on vaccine-derived polioviruses - worldwide, July 2012-December 2013
Abstract
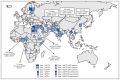
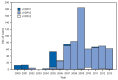
In 1988, the World Health Assembly resolved to eradicate poliomyelitis worldwide. One of the main tools used in polio eradication efforts has been live, attenuated oral poliovirus vaccine (OPV), an inexpensive vaccine easily administered by trained volunteers. OPV might require several doses to induce immunity, but then it provides long-term protection against paralytic disease through durable humoral immunity. Rare cases of vaccine-associated paralytic poliomyelitis can occur among immunologically normal OPV recipients, their contacts, and persons who are immunodeficient. In addition, vaccine-derived polioviruses (VDPVs) can emerge in areas with low OPV coverage to cause polio outbreaks and can replicate for years in persons who have primary, B-cell immunodeficiencies. This report updates previous surveillance summaries and describes VDPVs detected worldwide during July 2012-December 2013. Those include a new circulating VDPV (cVDPV) outbreak identified in Pakistan in 2012, with spread to Afghanistan; an outbreak in Afghanistan previously identified in 2009 that continued into 2013; a new outbreak in Chad that spread to Cameroon, Niger, and northeastern Nigeria; and an outbreak that began in Somalia in 2008 that continued and spread to Kenya in 2013. A large outbreak in Nigeria that was identified in 2005 was nearly stopped by the end of 2013. Additionally, 10 newly identified persons in eight countries were found to excrete immunodeficiency-associated VDPVs (iVDPVs), and VDPVs were found among immunocompetent persons and environmental samples in 13 countries. Because the majority of VDPV isolates are type 2, the World Health Organization has developed a plan for coordinated worldwide replacement of trivalent OPV (tOPV) with bivalent OPV (bOPV; types 1 and 3) by 2016, preceded by introduction of at least 1 dose of inactivated poliovirus vaccine (IPV) containing all three poliovirus serotypes into routine immunization schedules worldwide to ensure high population immunity to all polioviruses.
Figures
Similar articles
-
Update on Vaccine-Derived Polioviruses - Worldwide, January 2014-March 2015.MMWR Morb Mortal Wkly Rep. 2015 Jun 19;64(23):640-6. MMWR Morb Mortal Wkly Rep. 2015. PMID: 26086635 Free PMC article.
-
Update on vaccine-derived polioviruses--worldwide, April 2011-June 2012.MMWR Morb Mortal Wkly Rep. 2012 Sep 21;61:741-6. MMWR Morb Mortal Wkly Rep. 2012. PMID: 22992572
-
Update on vaccine-derived polioviruses--worldwide, July 2009-March 2011.MMWR Morb Mortal Wkly Rep. 2011 Jul 1;60(25):846-50. MMWR Morb Mortal Wkly Rep. 2011. PMID: 21716199
-
Vaccine-derived polioviruses.J Infect Dis. 2014 Nov 1;210 Suppl 1:S283-93. doi: 10.1093/infdis/jiu295. J Infect Dis. 2014. PMID: 25316847 Review.
-
Wild and vaccine-derived poliovirus circulation, and implications for polio eradication.Epidemiol Infect. 2017 Feb;145(3):413-419. doi: 10.1017/S0950268816002569. Epub 2016 Nov 21. Epidemiol Infect. 2017. PMID: 27866483 Free PMC article. Review.
Cited by
-
Genetic characterization and molecular evolution of type 3 vaccine-derived polioviruses from an immunodeficient patient in China.Virus Res. 2023 Sep;334:199177. doi: 10.1016/j.virusres.2023.199177. Epub 2023 Jul 24. Virus Res. 2023. PMID: 37479187 Free PMC article.
-
Achieving high immunogenicity against poliovirus with fractional doses of inactivated poliovirus vaccine in Ecuador-results from a cross-sectional serological survey.Lancet Reg Health Am. 2022 Jul;11:None. doi: 10.1016/j.lana.2022.100235. Lancet Reg Health Am. 2022. PMID: 35865654 Free PMC article.
-
Cost-Effectiveness of Three Poliovirus Immunization Schedules in Shanghai, China.Vaccines (Basel). 2021 Sep 23;9(10):1062. doi: 10.3390/vaccines9101062. Vaccines (Basel). 2021. PMID: 34696170 Free PMC article.
-
Update on Vaccine-Derived Poliovirus Outbreaks - Democratic Republic of the Congo and Horn of Africa, 2017-2018.MMWR Morb Mortal Wkly Rep. 2019 Mar 8;68(9):225-230. doi: 10.15585/mmwr.mm6809a2. MMWR Morb Mortal Wkly Rep. 2019. PMID: 30845121 Free PMC article.
-
Pediatric HIV Infection and Decreased Prevalence of OPV Point Mutations Linked to Vaccine-associated Paralytic Poliomyelitis.Clin Infect Dis. 2018 Oct 30;67(suppl_1):S78-S84. doi: 10.1093/cid/ciy635. Clin Infect Dis. 2018. PMID: 30376083 Free PMC article.
References
-
- CDC. Update on vaccine-derived polioviruses—worldwide, April 2011–June 2012. MMWR. 2012;61:741–6. - PubMed
-
- Global Polio Eradication Initiative. Polio eradication and endgame strategic plan 2013–2018. Geneva, Switzerland: World Health Organization, Global Polio Eradication Initiative; 2013. Available at http://www.polioeradication.org/portals/0/document/resources/strategywor....
-
- CDC. Tracking progress toward global polio eradication, 2010–2011. MMWR. 2012;61:265–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

