Tau protein modifications and interactions: their role in function and dysfunction
- PMID: 24646911
- PMCID: PMC3975420
- DOI: 10.3390/ijms15034671
Tau protein modifications and interactions: their role in function and dysfunction
Abstract
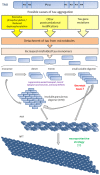
Tau protein is abundant in the central nervous system and involved in microtubule assembly and stabilization. It is predominantly associated with axonal microtubules and present at lower level in dendrites where it is engaged in signaling functions. Post-translational modifications of tau and its interaction with several proteins play an important regulatory role in the physiology of tau. As a consequence of abnormal modifications and expression, tau is redistributed from neuronal processes to the soma and forms toxic oligomers or aggregated deposits. The accumulation of tau protein is increasingly recognized as the neuropathological hallmark of a number of dementia disorders known as tauopathies. Dysfunction of tau protein may contribute to collapse of cytoskeleton, thereby causing improper anterograde and retrograde movement of motor proteins and their cargos on microtubules. These disturbances in intraneuronal signaling may compromise synaptic transmission as well as trophic support mechanisms in neurons.
Figures

Similar articles
-
Regulation of neuronal microtubule dynamics by tau: Implications for tauopathies.Int J Biol Macromol. 2019 Jul 15;133:473-483. doi: 10.1016/j.ijbiomac.2019.04.120. Epub 2019 Apr 17. Int J Biol Macromol. 2019. PMID: 31004638 Review.
-
Drosophila models of human tauopathies indicate that Tau protein toxicity in vivo is mediated by soluble cytosolic phosphorylated forms of the protein.J Neurochem. 2010 May;113(4):895-903. doi: 10.1111/j.1471-4159.2010.06663.x. Epub 2010 Feb 27. J Neurochem. 2010. PMID: 20193038
-
Tauopathies.Cell Mol Life Sci. 2007 Sep;64(17):2219-33. doi: 10.1007/s00018-007-7220-x. Cell Mol Life Sci. 2007. PMID: 17604998 Free PMC article. Review.
-
Non-Canonical Roles of Tau and Their Contribution to Synaptic Dysfunction.Int J Mol Sci. 2021 Sep 20;22(18):10145. doi: 10.3390/ijms221810145. Int J Mol Sci. 2021. PMID: 34576308 Free PMC article. Review.
-
Tau in physiology and pathology.Nat Rev Neurosci. 2016 Jan;17(1):5-21. doi: 10.1038/nrn.2015.1. Epub 2015 Dec 3. Nat Rev Neurosci. 2016. PMID: 26631930 Review.
Cited by
-
Novel model of secreted human tau protein reveals the impact of the abnormal N-glycosylation of tau on its aggregation propensity.Sci Rep. 2019 Feb 19;9(1):2254. doi: 10.1038/s41598-019-39218-x. Sci Rep. 2019. PMID: 30783169 Free PMC article.
-
Pathological Impact of Tau Proteolytical Process on Neuronal and Mitochondrial Function: a Crucial Role in Alzheimer's Disease.Mol Neurobiol. 2023 Oct;60(10):5691-5707. doi: 10.1007/s12035-023-03434-4. Epub 2023 Jun 19. Mol Neurobiol. 2023. PMID: 37332018 Review.
-
Nutrient-driven O-GlcNAc in proteostasis and neurodegeneration.J Neurochem. 2018 Jan;144(1):7-34. doi: 10.1111/jnc.14242. Epub 2017 Nov 20. J Neurochem. 2018. PMID: 29049853 Free PMC article. Review.
-
Protein modification in neurodegenerative diseases.MedComm (2020). 2024 Aug 4;5(8):e674. doi: 10.1002/mco2.674. eCollection 2024 Aug. MedComm (2020). 2024. PMID: 39105197 Free PMC article. Review.
-
Hyperphosphorylated Human Tau Accumulates at the Synapse, Localizing on Synaptic Mitochondrial Outer Membranes and Disrupting Respiration in a Mouse Model of Tauopathy.Front Mol Neurosci. 2022 Mar 10;15:852368. doi: 10.3389/fnmol.2022.852368. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35359570 Free PMC article.
References
-
- Ittner L.M., Ke Y.D., Delerue F., Bi M., Gladbach A., van Eersel J., Wölfing H., Chieng B.C., Christie M.J., Napier I.A., et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell. 2010;142:387–397. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

