Signalling profiles of H3 relaxin, H2 relaxin and R3(BΔ23-27)R/I5 acting at the relaxin family peptide receptor 3 (RXFP3)
- PMID: 24641548
- PMCID: PMC4243858
- DOI: 10.1111/bph.12623
Signalling profiles of H3 relaxin, H2 relaxin and R3(BΔ23-27)R/I5 acting at the relaxin family peptide receptor 3 (RXFP3)
Abstract
Background and purpose: Relaxin family peptide receptor 3 (RXFP3) is expressed in brain areas important for processing sensory information and feeding, suggesting that it may be a target for anti-anxiety and anti-obesity drugs. We examined the effects of H3 relaxin, the biased agonist H2 relaxin and the antagonist, R3(BΔ23-27)R/I5, on RXFP3 signalling to establish their suitability as tools to assess the physiological roles of RXFP3.
Experimental approach: The signalling profile of the RXFP3 ligands was determined using reporter gene assays, multiplexed signalling assays and direct examination of receptor-G protein and receptor-β-arrestin interactions using BRET.
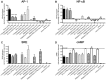
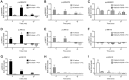
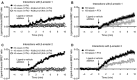
Key results: H2 relaxin activated p38MAPK and ERK1/2 with lower efficacy than H3 relaxin, but had similar efficacy for JNK1/2 phosphorylation. H2 or H3 relaxin activation of p38MAPK, JNK1/2 or ERK1/2 involved Pertussis toxin-sensitive G-proteins. R3(BΔ23-27)R/I5 blocked H3 relaxin AP-1 reporter gene activation, but not H2 relaxin AP-1 activation or H3 relaxin NF-κB activation. R3(BΔ23-27)R/I5 activated the SRE reporter, but did not inhibit either H2 or H3 relaxin SRE activation. R3(BΔ23-27)R/I5 blocked H3 relaxin-stimulated p38MAPK and ERK1/2 phosphorylation, but was a weak partial agonist for p38MAPK and ERK1/2 signalling. p38MAPK activation by R3(BΔ23-27)R/I5 was G protein-independent. H3 relaxin-activated RXFP3 interacts with Gαi2 , Gαi3 , Gαo A and Gαo B whereas H2 relaxin or R3(BΔ23-27)R/I5 induce interactions only with Gαi2 or Gαo B . Only H3 relaxin promoted RXFP3/β-arrestin interactions that were blocked by R3(BΔ23-27)R/I5.
Conclusion and implications: Understanding signalling profile of drugs acting at RXFP3 is essential for development of therapies targeting this receptor.
Keywords: R3(BΔ23-27)R/I5; RXFP3 signalling; relaxin.
© 2014 The British Pharmacological Society.
Figures




Similar articles
-
The structural and functional role of the B-chain C-terminal arginine in the relaxin-3 peptide antagonist, R3(BDelta23-27)R/I5.Chem Biol Drug Des. 2009 Jan;73(1):46-52. doi: 10.1111/j.1747-0285.2008.00756.x. Chem Biol Drug Des. 2009. PMID: 19152634
-
Partial agonist activity of R3(BΔ23-27)R/I5 at RXFP3--investigation of in vivo and in vitro pharmacology.Eur J Pharmacol. 2015 Jan 15;747:123-31. doi: 10.1016/j.ejphar.2014.11.041. Epub 2014 Dec 11. Eur J Pharmacol. 2015. PMID: 25496752
-
R3(BDelta23 27)R/I5 chimeric peptide, a selective antagonist for GPCR135 and GPCR142 over relaxin receptor LGR7: in vitro and in vivo characterization.J Biol Chem. 2007 Aug 31;282(35):25425-35. doi: 10.1074/jbc.M701416200. Epub 2007 Jul 2. J Biol Chem. 2007. PMID: 17606621
-
Relaxin-3, INSL5, and their receptors.Results Probl Cell Differ. 2008;46:213-37. doi: 10.1007/400_2007_055. Results Probl Cell Differ. 2008. PMID: 18236022 Review.
-
Relaxin family peptide receptors--former orphans reunite with their parent ligands to activate multiple signalling pathways.Br J Pharmacol. 2007 Mar;150(6):677-91. doi: 10.1038/sj.bjp.0707140. Epub 2007 Feb 12. Br J Pharmacol. 2007. PMID: 17293890 Free PMC article. Review.
Cited by
-
A single-chain derivative of the relaxin hormone is a functionally selective agonist of the G protein-coupled receptor, RXFP1.Chem Sci. 2016 Jun 1;7(6):3805-3819. doi: 10.1039/c5sc04754d. Epub 2016 Feb 26. Chem Sci. 2016. PMID: 30155023 Free PMC article.
-
High-Throughput Screening Campaign Identified a Potential Small Molecule RXFP3/4 Agonist.Molecules. 2021 Dec 11;26(24):7511. doi: 10.3390/molecules26247511. Molecules. 2021. PMID: 34946593 Free PMC article.
-
Real-time examination of cAMP activity at relaxin family peptide receptors using a BRET-based biosensor.Pharmacol Res Perspect. 2018 Sep 24;6(5):e00432. doi: 10.1002/prp2.432. eCollection 2018 Oct. Pharmacol Res Perspect. 2018. PMID: 30263124 Free PMC article.
-
Targeting the relaxin-3/RXFP3 system: a patent review for the last two decades.Expert Opin Ther Pat. 2024 Jan-Feb;34(1-2):71-81. doi: 10.1080/13543776.2024.2338099. Epub 2024 Apr 4. Expert Opin Ther Pat. 2024. PMID: 38573177 Review.
-
Discovery and Characterization of the First Nonpeptide Antagonists for the Relaxin-3/RXFP3 System.J Med Chem. 2022 Jun 9;65(11):7959-7974. doi: 10.1021/acs.jmedchem.2c00508. Epub 2022 May 20. J Med Chem. 2022. PMID: 35594150 Free PMC article.
References
-
- Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004;279:35518–35525. - PubMed
-
- Baker JG, Hall IP, Hill SJ. Agonist and inverse agonist actions of beta-blockers at the human beta 2-adrenoceptor provide evidence for agonist-directed signaling. Mol Pharmacol. 2003;64:1357–1369. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

