Playing hide and seek: how glycosylation of the influenza virus hemagglutinin can modulate the immune response to infection
- PMID: 24638204
- PMCID: PMC3970151
- DOI: 10.3390/v6031294
Playing hide and seek: how glycosylation of the influenza virus hemagglutinin can modulate the immune response to infection
Abstract
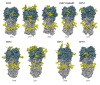
Seasonal influenza A viruses (IAV) originate from pandemic IAV and have undergone changes in antigenic structure, including addition of glycans to the hemagglutinin (HA) glycoprotein. The viral HA is the major target recognized by neutralizing antibodies and glycans have been proposed to shield antigenic sites on HA, thereby promoting virus survival in the face of widespread vaccination and/or infection. However, addition of glycans can also interfere with the receptor binding properties of HA and this must be compensated for by additional mutations, creating a fitness barrier to accumulation of glycosylation sites. In addition, glycans on HA are also recognized by phylogenetically ancient lectins of the innate immune system and the benefit provided by evasion of humoral immunity is balanced by attenuation of infection. Therefore, a fine balance must exist regarding the optimal pattern of HA glycosylation to offset competing pressures associated with recognition by innate defenses, evasion of humoral immunity and maintenance of virus fitness. In this review, we examine HA glycosylation patterns of IAV associated with pandemic and seasonal influenza and discuss recent advancements in our understanding of interactions between IAV glycans and components of innate and adaptive immunity.
Figures
Similar articles
-
Addition of glycosylation to influenza A virus hemagglutinin modulates antibody-mediated recognition of H1N1 2009 pandemic viruses.J Immunol. 2013 Mar 1;190(5):2169-77. doi: 10.4049/jimmunol.1202433. Epub 2013 Jan 30. J Immunol. 2013. PMID: 23365085
-
Specific sites of N-linked glycosylation on the hemagglutinin of H1N1 subtype influenza A virus determine sensitivity to inhibitors of the innate immune system and virulence in mice.J Immunol. 2011 Aug 15;187(4):1884-94. doi: 10.4049/jimmunol.1100295. Epub 2011 Jul 18. J Immunol. 2011. PMID: 21768397
-
N-linked glycosylation in the hemagglutinin of influenza A viruses.Yonsei Med J. 2012 Sep;53(5):886-93. doi: 10.3349/ymj.2012.53.5.886. Yonsei Med J. 2012. PMID: 22869469 Free PMC article. Review.
-
A Perspective on the Structural and Functional Constraints for Immune Evasion: Insights from Influenza Virus.J Mol Biol. 2017 Aug 18;429(17):2694-2709. doi: 10.1016/j.jmb.2017.06.015. Epub 2017 Jun 23. J Mol Biol. 2017. PMID: 28648617 Free PMC article. Review.
-
Innate Sensing of Influenza A Virus Hemagglutinin Glycoproteins by the Host Endoplasmic Reticulum (ER) Stress Pathway Triggers a Potent Antiviral Response via ER-Associated Protein Degradation.J Virol. 2017 Dec 14;92(1):e01690-17. doi: 10.1128/JVI.01690-17. Print 2018 Jan 1. J Virol. 2017. PMID: 29046440 Free PMC article.
Cited by
-
The Chinese Hamster Ovary Cell-Based H9 HA Subunit Avian Influenza Vaccine Provides Complete Protection against the H9N2 Virus Challenge in Chickens.Viruses. 2024 Jan 22;16(1):163. doi: 10.3390/v16010163. Viruses. 2024. PMID: 38275973 Free PMC article.
-
Pandemic Swine H1N1 Influenza Viruses with Almost Undetectable Neuraminidase Activity Are Not Transmitted via Aerosols in Ferrets and Are Inhibited by Human Mucus but Not Swine Mucus.J Virol. 2015 Jun;89(11):5935-48. doi: 10.1128/JVI.02537-14. Epub 2015 Mar 25. J Virol. 2015. PMID: 25810540 Free PMC article.
-
Continuing evolution of H6N2 influenza a virus in South African chickens and the implications for diagnosis and control.BMC Vet Res. 2019 Dec 18;15(1):455. doi: 10.1186/s12917-019-2210-4. BMC Vet Res. 2019. PMID: 31852473 Free PMC article.
-
Breathing and Tilting: Mesoscale Simulations Illuminate Influenza Glycoprotein Vulnerabilities.ACS Cent Sci. 2022 Dec 28;8(12):1646-1663. doi: 10.1021/acscentsci.2c00981. Epub 2022 Dec 8. ACS Cent Sci. 2022. PMID: 36589893 Free PMC article.
-
Accelerating Bayesian inference of dependency between mixed-type biological traits.PLoS Comput Biol. 2023 Aug 28;19(8):e1011419. doi: 10.1371/journal.pcbi.1011419. eCollection 2023 Aug. PLoS Comput Biol. 2023. PMID: 37639445 Free PMC article.
References
-
- Deom C.M., Schulze I.T. Oligosaccharide composition of an influenza virus hemagglutinin with host-determined binding properties. J. Biol. Chem. 1985;260:14771–14774. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

