Both contractile axial and lateral traction force dynamics drive amoeboid cell motility
- PMID: 24637328
- PMCID: PMC3998796
- DOI: 10.1083/jcb.201307106
Both contractile axial and lateral traction force dynamics drive amoeboid cell motility
Abstract
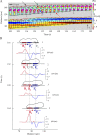
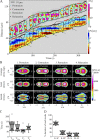
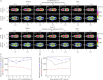
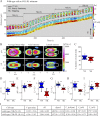
Chemotaxing Dictyostelium discoideum cells adapt their morphology and migration speed in response to intrinsic and extrinsic cues. Using Fourier traction force microscopy, we measured the spatiotemporal evolution of shape and traction stresses and constructed traction tension kymographs to analyze cell motility as a function of the dynamics of the cell's mechanically active traction adhesions. We show that wild-type cells migrate in a step-wise fashion, mainly forming stationary traction adhesions along their anterior-posterior axes and exerting strong contractile axial forces. We demonstrate that lateral forces are also important for motility, especially for migration on highly adhesive substrates. Analysis of two mutant strains lacking distinct actin cross-linkers (mhcA(-) and abp120(-) cells) on normal and highly adhesive substrates supports a key role for lateral contractions in amoeboid cell motility, whereas the differences in their traction adhesion dynamics suggest that these two strains use distinct mechanisms to achieve migration. Finally, we provide evidence that the above patterns of migration may be conserved in mammalian amoeboid cells.
Figures


Similar articles
-
The SCAR/WAVE complex is necessary for proper regulation of traction stresses during amoeboid motility.Mol Biol Cell. 2011 Nov;22(21):3995-4003. doi: 10.1091/mbc.E11-03-0278. Epub 2011 Sep 7. Mol Biol Cell. 2011. PMID: 21900496 Free PMC article.
-
Distribution of traction forces associated with shape changes during amoeboid cell migration.Annu Int Conf IEEE Eng Med Biol Soc. 2009;2009:3346-9. doi: 10.1109/IEMBS.2009.5333191. Annu Int Conf IEEE Eng Med Biol Soc. 2009. PMID: 19964075 Free PMC article.
-
Mechanosensitive Adhesion Explains Stepping Motility in Amoeboid Cells.Biophys J. 2017 Jun 20;112(12):2672-2682. doi: 10.1016/j.bpj.2017.04.033. Biophys J. 2017. PMID: 28636923 Free PMC article.
-
Amoeboid leukocyte crawling through extracellular matrix: lessons from the Dictyostelium paradigm of cell movement.J Leukoc Biol. 2001 Oct;70(4):491-509. J Leukoc Biol. 2001. PMID: 11590185 Review.
-
Forces in cell locomotion.Biochem Soc Symp. 1999;65:299-314. Biochem Soc Symp. 1999. PMID: 10320946 Review.
Cited by
-
Three-dimensional balance of cortical tension and axial contractility enables fast amoeboid migration.Biophys J. 2015 Feb 17;108(4):821-832. doi: 10.1016/j.bpj.2014.11.3478. Biophys J. 2015. PMID: 25692587 Free PMC article.
-
Integration of Extracellular Matrices into Organ-on-Chip Systems.Adv Healthc Mater. 2023 Aug;12(20):e2203256. doi: 10.1002/adhm.202203256. Epub 2023 Apr 29. Adv Healthc Mater. 2023. PMID: 37018430 Free PMC article. Review.
-
Mechanical competition triggered by innate immune signaling drives the collective extrusion of bacterially infected epithelial cells.Dev Cell. 2021 Feb 22;56(4):443-460.e11. doi: 10.1016/j.devcel.2021.01.012. Dev Cell. 2021. PMID: 33621492 Free PMC article.
-
Mechanical stress and network structure drive protein dynamics during cytokinesis.Curr Biol. 2015 Mar 2;25(5):663-70. doi: 10.1016/j.cub.2015.01.025. Epub 2015 Feb 19. Curr Biol. 2015. PMID: 25702575 Free PMC article.
-
Mechanical Forces Govern Interactions of Host Cells with Intracellular Bacterial Pathogens.Microbiol Mol Biol Rev. 2022 Jun 15;86(2):e0009420. doi: 10.1128/mmbr.00094-20. Epub 2022 Mar 14. Microbiol Mol Biol Rev. 2022. PMID: 35285720 Free PMC article. Review.
References
-
- Alonso-Latorre B. 2010. Force and shape coordination in amoeboid cell motility. PhD thesis University of California, San Diego: 152 pp
-
- Bagorda A., Mihaylov V.A., Parent C.A. 2006. Chemotaxis: moving forward and holding on to the past. Thromb. Haemost. 95:12–21 - PubMed
-
- Balaban N.Q., Schwarz U.S., Riveline D., Goichberg P., Tzur G., Sabanay I., Mahalu D., Safran S., Bershadsky A.D., Addadi L., Geiger B. 2001. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 3:466–472 10.1038/35074532 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

