Cytomegalovirus hijacks CX3CR1(hi) patrolling monocytes as immune-privileged vehicles for dissemination in mice
- PMID: 24629341
- PMCID: PMC3989205
- DOI: 10.1016/j.chom.2014.02.002
Cytomegalovirus hijacks CX3CR1(hi) patrolling monocytes as immune-privileged vehicles for dissemination in mice
Abstract
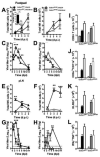
Peripheral blood myelomonocytic cells are important for cytomegalovirus dissemination to distal organs such as salivary glands where persistent replication and shedding dictates transmission patterns. We find that this process is markedly enhanced by the murine cytomegalovirus (MCMV)-encoded CC chemokine, MCK2, which promotes recruitment of CX3CR1(hi) patrolling monocytes to initial infection sites in the mouse. There, these cells become infected and traffic via the bloodstream to distal sites. In contrast, inflammatory monocytes, the other major myelomonocytic subset, remain virus negative. CX3CR1 deficiency prevents patrolling monocyte migration on the vascular endothelium and interrupts MCMV dissemination to the salivary glands independent of antiviral NK and T cell immune control. In this manner, CX3CR1(hi) patrolling monocytes serve as immune-privileged vehicles to transport MCMV via the bloodstream to distal organs. MCMV commandeers patrolling monocytes to mediate systemic infection and seed a persistent reservoir essential for horizontal transmission.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
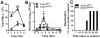
Similar articles
-
Murine cytomegalovirus disseminates independently of CX3CR1, CCL2 or its m131/m129 chemokine homologue.J Gen Virol. 2019 Dec;100(12):1695-1700. doi: 10.1099/jgv.0.001333. Epub 2019 Oct 11. J Gen Virol. 2019. PMID: 31609196
-
Murine Cytomegalovirus MCK-2 Facilitates In Vivo Infection Transfer from Dendritic Cells to Salivary Gland Acinar Cells.J Virol. 2021 Aug 10;95(17):e0069321. doi: 10.1128/JVI.00693-21. Epub 2021 Aug 10. J Virol. 2021. PMID: 34132572 Free PMC article.
-
Dissecting the cytomegalovirus CC chemokine: Chemokine activity and gHgLchemokine-dependent cell tropism are independent players in CMV infection.PLoS Pathog. 2023 Dec 8;19(12):e1011793. doi: 10.1371/journal.ppat.1011793. eCollection 2023 Dec. PLoS Pathog. 2023. PMID: 38064525 Free PMC article.
-
The salivary glands as a privileged site of cytomegalovirus immune evasion and persistence.Med Microbiol Immunol. 2008 Jun;197(2):205-13. doi: 10.1007/s00430-008-0077-2. Epub 2008 Feb 8. Med Microbiol Immunol. 2008. PMID: 18259775 Review.
-
Caspase-8-dependent control of NK- and T cell responses during cytomegalovirus infection.Med Microbiol Immunol. 2019 Aug;208(3-4):555-571. doi: 10.1007/s00430-019-00616-7. Epub 2019 May 16. Med Microbiol Immunol. 2019. PMID: 31098689 Review.
Cited by
-
Cytomegalovirus immune evasion of myeloid lineage cells.Med Microbiol Immunol. 2015 Jun;204(3):367-82. doi: 10.1007/s00430-015-0403-4. Epub 2015 Mar 17. Med Microbiol Immunol. 2015. PMID: 25776081 Review.
-
Necroptosis-based CRISPR knockout screen reveals Neuropilin-1 as a critical host factor for early stages of murine cytomegalovirus infection.Proc Natl Acad Sci U S A. 2020 Aug 18;117(33):20109-20116. doi: 10.1073/pnas.1921315117. Epub 2020 Aug 3. Proc Natl Acad Sci U S A. 2020. PMID: 32747526 Free PMC article.
-
Mouse Cytomegalovirus Differentially Exploits Cell Surface Glycosaminoglycans in a Cell Type-Dependent and MCK-2-Independent Manner.Viruses. 2019 Dec 27;12(1):31. doi: 10.3390/v12010031. Viruses. 2019. PMID: 31892128 Free PMC article.
-
Roles of GP33, a guinea pig cytomegalovirus-encoded G protein-coupled receptor homolog, in cellular signaling, viral growth and inflammation in vitro and in vivo.PLoS Pathog. 2018 Dec 20;14(12):e1007487. doi: 10.1371/journal.ppat.1007487. eCollection 2018 Dec. PLoS Pathog. 2018. PMID: 30571759 Free PMC article.
-
Human cytomegalovirus modulates monocyte-mediated innate immune responses during short-term experimental latency in vitro.J Virol. 2014 Aug;88(16):9391-405. doi: 10.1128/JVI.00934-14. Epub 2014 Jun 11. J Virol. 2014. PMID: 24920803 Free PMC article.
References
-
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. - PubMed
-
- Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

