Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium
- PMID: 24625784
- PMCID: PMC4017349
- DOI: 10.1161/CIRCRESAHA.114.303204
Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium
Abstract
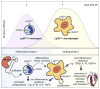
Rationale: Healing after myocardial infarction involves the biphasic accumulation of inflammatory lymphocyte antigen 6C (Ly-6C)(high) and reparative Ly-6C(low) monocytes/macrophages (Mo/MΦ). According to 1 model, Mo/MΦ heterogeneity in the heart originates in the blood and involves the sequential recruitment of distinct monocyte subsets that differentiate to distinct macrophages. Alternatively, heterogeneity may arise in tissue from 1 circulating subset via local macrophage differentiation and polarization. The orphan nuclear hormone receptor, nuclear receptor subfamily 4, group a, member 1 (Nr4a1), is essential to Ly-6C(low) monocyte production but dispensable to Ly-6C(low) macrophage differentiation; dependence on Nr4a1 can thus discriminate between systemic and local origins of macrophage heterogeneity.
Objective: This study tested the role of Nr4a1 in myocardial infarction in the context of the 2 Mo/MΦ accumulation scenarios.
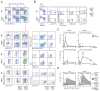
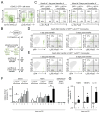
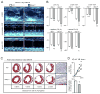
Methods and results: We show that Ly-6C(high) monocytes infiltrate the infarcted myocardium and, unlike Ly-6C(low) monocytes, differentiate to cardiac macrophages. In the early, inflammatory phase of acute myocardial ischemic injury, Ly-6C(high) monocytes accrue in response to a brief C-C chemokine ligand 2 burst. In the second, reparative phase, accumulated Ly-6C(high) monocytes give rise to reparative Ly-6C(low) F4/80(high) macrophages that proliferate locally. In the absence of Nr4a1, Ly-6C(high) monocytes express heightened levels of C-C chemokine receptor 2 on their surface, avidly infiltrate the myocardium, and differentiate to abnormally inflammatory macrophages, which results in defective healing and compromised heart function.
Conclusions: Ly-6C(high) monocytes orchestrate both inflammatory and reparative phases during myocardial infarction and depend on Nr4a1 to limit their influx and inflammatory cytokine expression.
Keywords: hormone receptors; macrophages; monocytes; myocardial infarction; nuclear.
Figures




Comment in
-
It takes two to tango: monocyte and macrophage duality in the infarcted heart.Circ Res. 2014 May 9;114(10):1558-60. doi: 10.1161/CIRCRESAHA.114.303933. Circ Res. 2014. PMID: 24812348 Free PMC article. No abstract available.
Similar articles
-
It takes two to tango: monocyte and macrophage duality in the infarcted heart.Circ Res. 2014 May 9;114(10):1558-60. doi: 10.1161/CIRCRESAHA.114.303933. Circ Res. 2014. PMID: 24812348 Free PMC article. No abstract available.
-
Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis.J Am Coll Cardiol. 2010 Apr 13;55(15):1629-38. doi: 10.1016/j.jacc.2009.08.089. J Am Coll Cardiol. 2010. PMID: 20378083 Free PMC article.
-
Topiramate modulates post-infarction inflammation primarily by targeting monocytes or macrophages.Cardiovasc Res. 2017 Apr 1;113(5):475-487. doi: 10.1093/cvr/cvx027. Cardiovasc Res. 2017. PMID: 28339742
-
Monocytes, Macrophages and Other Inflammatory Mediators of Abdominal Aortic Aneurysm.Curr Pharm Des. 2015;21(28):4007-15. doi: 10.2174/1381612821666150826093855. Curr Pharm Des. 2015. PMID: 26306839 Review.
-
Monocyte and macrophage dynamics during atherogenesis.Arterioscler Thromb Vasc Biol. 2011 Jul;31(7):1506-16. doi: 10.1161/ATVBAHA.110.221127. Arterioscler Thromb Vasc Biol. 2011. PMID: 21677293 Free PMC article. Review.
Cited by
-
Ginsenoside Rd Promotes Cardiac Repair After Myocardial Infarction by Modulating Monocytes/Macrophages Subsets Conversion.Drug Des Devel Ther. 2022 Aug 22;16:2767-2782. doi: 10.2147/DDDT.S377624. eCollection 2022. Drug Des Devel Ther. 2022. PMID: 36033133 Free PMC article.
-
Nuclear receptor 4A (NR4A) family - orphans no more.J Steroid Biochem Mol Biol. 2016 Mar;157:48-60. doi: 10.1016/j.jsbmb.2015.04.016. Epub 2015 Apr 23. J Steroid Biochem Mol Biol. 2016. PMID: 25917081 Free PMC article.
-
Nonclassical patrolling monocyte function in the vasculature.Arterioscler Thromb Vasc Biol. 2015 Jun;35(6):1306-16. doi: 10.1161/ATVBAHA.114.304650. Epub 2015 Apr 2. Arterioscler Thromb Vasc Biol. 2015. PMID: 25838429 Free PMC article. Review.
-
Macrophages in the Remodeling Failing Heart.Circ Res. 2016 Sep 16;119(7):776-8. doi: 10.1161/CIRCRESAHA.116.309624. Circ Res. 2016. PMID: 27635078 Free PMC article. No abstract available.
-
Single-cell transcriptomics identifies the differentiation trajectory from inflammatory monocytes to pro-resolving macrophages in a mouse skin allergy model.Nat Commun. 2024 Feb 23;15(1):1666. doi: 10.1038/s41467-024-46148-4. Nat Commun. 2024. PMID: 38396021 Free PMC article.
References
-
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De Leon FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. - PMC - PubMed
-
- Bloom DE, Cafiero ET, Jane-Llopis E, Abrahams-Gessel S, Bloom LR, Fathima S, Feigl AB, Gaziano T, Mowafi M, Pandya A, Prettner K, Rosenberg L, Seligman B, Stein AZ, Weinstein C. The Global Economic Burden of Noncommunicable Diseases. Geneva: World Economic Forum; 2011.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

