Cerebrospinal fluid α-synuclein predicts cognitive decline in Parkinson disease progression in the DATATOP cohort
- PMID: 24625392
- PMCID: PMC3969999
- DOI: 10.1016/j.ajpath.2013.12.007
Cerebrospinal fluid α-synuclein predicts cognitive decline in Parkinson disease progression in the DATATOP cohort
Abstract
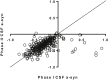
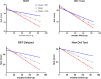
Most patients with Parkinson disease (PD) develop both cognitive and motor impairment, and biomarkers for progression are urgently needed. Although α-synuclein is altered in cerebrospinal fluid of patients with PD, it is not known whether it predicts motor or cognitive deterioration. We examined clinical data and α-synuclein in >300 unmedicated patients with PD who participated in the deprenyl and tocopherol antioxidative therapy of parkinsonism (DATATOP) study, with up to 8 years of follow-up. Longitudinal measures of motor and cognitive function were studied before (phase 1) and during (phase 2) levodopa therapy; cerebrospinal fluid was collected at the beginning of each phase. Correlations and linear mixed models were used to assess α-synuclein association with disease severity and prediction of progression in the subsequent follow-up period. Despite decreasing α-synuclein (phase 1 to phase 2 change of -0.05 ± 0.21 log-transformed values, P < 0.001), no correlations were observed between α-synuclein and motor symptoms. Longitudinally, lower α-synuclein predicted better preservation of cognitive function by several measures [Selective Reminding Test total recall α-synuclein × time interaction effect coefficient, -0.12 (P = 0.037); delayed recall, -0.05 (P = 0.002); New Dot Test, -0.03 (P = 0.002)]. Thus, α-synuclein, although not clinically useful for motor progression, might predict cognitive decline, and future longitudinal studies should include this outcome for further validation.
Copyright © 2014 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
CSF tau and tau/Aβ42 predict cognitive decline in Parkinson's disease.Parkinsonism Relat Disord. 2015 Mar;21(3):271-6. doi: 10.1016/j.parkreldis.2014.12.027. Epub 2015 Jan 5. Parkinsonism Relat Disord. 2015. PMID: 25596881 Free PMC article. Clinical Trial.
-
REM behavior disorder predicts motor progression and cognitive decline in Parkinson disease.Neurology. 2018 Sep 4;91(10):e894-e905. doi: 10.1212/WNL.0000000000006134. Epub 2018 Aug 8. Neurology. 2018. PMID: 30089615
-
Longitudinal changes in CSF alpha-synuclein species reflect Parkinson's disease progression.Mov Disord. 2016 Oct;31(10):1535-1542. doi: 10.1002/mds.26754. Mov Disord. 2016. PMID: 27548849
-
DATATOP: a decade of neuroprotective inquiry. Parkinson Study Group. Deprenyl And Tocopherol Antioxidative Therapy Of Parkinsonism.Ann Neurol. 1998 Sep;44(3 Suppl 1):S160-6. Ann Neurol. 1998. PMID: 9749589 Review.
-
Do CSF Biomarkers Predict Progression to Cognitive Impairment in Parkinson's disease patients? A Systematic Review.Neuropsychol Rev. 2015 Dec;25(4):411-23. doi: 10.1007/s11065-015-9307-8. Epub 2015 Dec 1. Neuropsychol Rev. 2015. PMID: 26626621 Free PMC article. Review.
Cited by
-
Alpha Synuclein Toxicity and Non-Motor Parkinson's.Cells. 2024 Jul 27;13(15):1265. doi: 10.3390/cells13151265. Cells. 2024. PMID: 39120295 Free PMC article. Review.
-
Diagnostic Values of Cerebrospinal Fluid T-Tau and Aβ₄₂ using Meso Scale Discovery Assays for Alzheimer's Disease.J Alzheimers Dis. 2015;45(3):709-19. doi: 10.3233/JAD-143099. J Alzheimers Dis. 2015. PMID: 25613100 Free PMC article.
-
Cardiovascular dysautonomia and cognitive impairment in Parkinson's disease (Review).Med Int (Lond). 2024 Sep 19;4(6):70. doi: 10.3892/mi.2024.194. eCollection 2024 Nov-Dec. Med Int (Lond). 2024. PMID: 39355336 Free PMC article. Review.
-
Onset of Mild Cognitive Impairment in Parkinson Disease.Alzheimer Dis Assoc Disord. 2016 Apr-Jun;30(2):127-33. doi: 10.1097/WAD.0000000000000088. Alzheimer Dis Assoc Disord. 2016. PMID: 25850732 Free PMC article.
-
The Future of Targeted Gene-Based Treatment Strategies and Biomarkers in Parkinson's Disease.Biomolecules. 2020 Jun 16;10(6):912. doi: 10.3390/biom10060912. Biomolecules. 2020. PMID: 32560161 Free PMC article. Review.
References
-
- Buter T.C., van den Hout A., Matthews F.E., Larsen J.P., Brayne C., Aarsland D. Dementia and survival in Parkinson disease: a 12-year population study. Neurology. 2008;70:1017–1022. - PubMed
-
- Pagonabarraga J., Kulisevsky J. Cognitive impairment and dementia in Parkinson’s disease. Neurobiol Dis. 2012;46:590–596. - PubMed
-
- Satake W., Nakabayashi Y., Mizuta I., Hirota Y., Ito C., Kubo M., Kawaguchi T., Tsunoda T., Watanabe M., Takeda A., Tomiyama H., Nakashima K., Hasegawa K., Obata F., Yoshikawa T., Kawakami H., Sakoda S., Yamamoto M., Hattori N., Murata M., Nakamura Y., Toda T. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat Genet. 2009;41:1303–1307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- ES016873/ES/NIEHS NIH HHS/United States
- NS082137/NS/NINDS NIH HHS/United States
- P42 ES004696/ES/NIEHS NIH HHS/United States
- R21 NS060252/NS/NINDS NIH HHS/United States
- T32 ES015459/ES/NIEHS NIH HHS/United States
- P30 ES007033/ES/NIEHS NIH HHS/United States
- R01 AG033398/AG/NIA NIH HHS/United States
- ES004696-5897/ES/NIEHS NIH HHS/United States
- U01 NS082137/NS/NINDS NIH HHS/United States
- NS062684-6221/NS/NINDS NIH HHS/United States
- NS060252/NS/NINDS NIH HHS/United States
- AG033398/AG/NIA NIH HHS/United States
- R01 NS057567/NS/NINDS NIH HHS/United States
- ES007033-6364/ES/NIEHS NIH HHS/United States
- NS057567/NS/NINDS NIH HHS/United States
- R01 ES019277/ES/NIEHS NIH HHS/United States
- R01 ES016873/ES/NIEHS NIH HHS/United States
- T32ES015459/ES/NIEHS NIH HHS/United States
- ES019277/ES/NIEHS NIH HHS/United States
- P50 NS062684/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

