Negative regulation of Hif1a expression and TH17 differentiation by the hypoxia-regulated microRNA miR-210
- PMID: 24608041
- PMCID: PMC3996831
- DOI: 10.1038/ni.2846
Negative regulation of Hif1a expression and TH17 differentiation by the hypoxia-regulated microRNA miR-210
Abstract
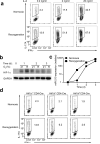
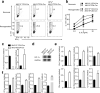
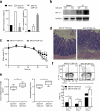
The microRNA miR-210 is a signature of hypoxia. We found robust increase in the abundance of miR-210 (>100-fold) in activated T cells, especially in the TH17 lineage of helper T cells. Hypoxia acted in synergy with stimulation via the T cell antigen receptor (TCR) and coreceptor CD28 to accelerate and increase Mir210 expression. Mir210 was directly regulated by HIF-1α, a key transcriptional regulator of TH17 polarization. Unexpectedly, we identified Hif1a as a target of miR-210, which suggested negative feedback by miR-210 in inhibiting HIF-1α expression. Deletion of Mir210 promoted TH17 differentiation under conditions of limited oxygen. In experimental colitis, miR-210 reduced the abundance of Hif1a transcripts and the proportion of cells that produced inflammatory cytokines and controlled disease severity. Our study identifies miR-210 as an important regulator of T cell differentiation in hypoxia, which can limit immunopathology.
Figures

Similar articles
-
HIF-1α and miR-210 differential and lineage-specific expression in systemic lupus erythematosus.Mol Immunol. 2021 May;133:128-134. doi: 10.1016/j.molimm.2021.02.019. Epub 2021 Feb 28. Mol Immunol. 2021. PMID: 33657462 Free PMC article.
-
The hypoxia-inducible miR-429 regulates hypoxia-inducible factor-1α expression in human endothelial cells through a negative feedback loop.FASEB J. 2015 Apr;29(4):1467-79. doi: 10.1096/fj.14-267054. Epub 2014 Dec 30. FASEB J. 2015. PMID: 25550463 Free PMC article.
-
A Distinct Inhibitory Function for miR-18a in Th17 Cell Differentiation.J Immunol. 2017 Jul 15;199(2):559-569. doi: 10.4049/jimmunol.1700170. Epub 2017 Jun 12. J Immunol. 2017. PMID: 28607111 Free PMC article.
-
HIF-1α (Hypoxia-Inducible Factor-1α) Promotes Macrophage Necroptosis by Regulating miR-210 and miR-383.Arterioscler Thromb Vasc Biol. 2020 Mar;40(3):583-596. doi: 10.1161/ATVBAHA.119.313290. Epub 2020 Jan 30. Arterioscler Thromb Vasc Biol. 2020. PMID: 31996026
-
Dysregulated MicroRNA Involvement in Multiple Sclerosis by Induction of T Helper 17 Cell Differentiation.Front Immunol. 2018 Jun 4;9:1256. doi: 10.3389/fimmu.2018.01256. eCollection 2018. Front Immunol. 2018. PMID: 29915595 Free PMC article. Review.
Cited by
-
Hypoxia-inducible factors in cancer stem cells and inflammation.Trends Pharmacol Sci. 2015 Jun;36(6):374-83. doi: 10.1016/j.tips.2015.03.003. Epub 2015 Apr 6. Trends Pharmacol Sci. 2015. PMID: 25857287 Free PMC article. Review.
-
Research on the Protective Effect of MiR-185-3p Mediated by Huangqin-Tang Decoction (HQT) on the Epithelial Barrier Function of Ulcerative Colitis.Evid Based Complement Alternat Med. 2021 Dec 21;2021:4775606. doi: 10.1155/2021/4775606. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34970325 Free PMC article.
-
Emerging Role of Non-Coding RNAs in Regulation of T-Lymphocyte Function.Front Immunol. 2021 Nov 4;12:756042. doi: 10.3389/fimmu.2021.756042. eCollection 2021. Front Immunol. 2021. PMID: 34804042 Free PMC article. Review.
-
PCR Array Profiling of miRNA Expression Involved in the Differentiation of Amniotic Fluid Stem Cells toward Endothelial and Smooth Muscle Progenitor Cells.Int J Mol Sci. 2023 Dec 25;25(1):302. doi: 10.3390/ijms25010302. Int J Mol Sci. 2023. PMID: 38203477 Free PMC article.
-
Sugar, fat, and protein: new insights into what T cells crave.Curr Opin Immunol. 2015 Apr;33:49-54. doi: 10.1016/j.coi.2015.01.015. Epub 2015 Feb 6. Curr Opin Immunol. 2015. PMID: 25665466 Free PMC article. Review.
References
-
- Caldwell CC, et al. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J. Immunol. 2001;167:6140–6149. - PubMed
-
- Bedogni B, et al. The hypoxic microenvironment of the skin contributes to Akt-mediated melanocyte transformation. Cancer Cell. 2005;8:443–454. - PubMed
-
- Taylor CT, Colgan SP. Hypoxia and gastrointestinal disease. J. Mol. Med. 2007;85:1295–1300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

