Therapeutic vaccines and cancer: focus on DPX-0907
- PMID: 24596453
- PMCID: PMC3930480
- DOI: 10.2147/BTT.S55196
Therapeutic vaccines and cancer: focus on DPX-0907
Abstract
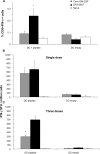
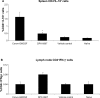
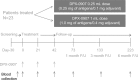
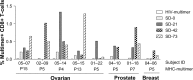
In an attempt to significantly enhance immunogenicity of peptide cancer vaccines, we developed a novel non-emulsion depot-forming vaccine platform called DepoVax™ (DPX). Human leukocyte antigen (HLA)-A2 restricted peptides naturally presented by cancer cells were used as antigens to create a therapeutic cancer vaccine, DPX-0907. In a phase I clinical study, the safety and immune-activating potential of DPX-0907 in advanced-stage breast, ovarian, and prostate cancer patients were examined, following encouraging results in HLA-A2 transgenic mice. The DPX-0907 vaccine was shown to be safe and well tolerated, with injection-site reactions being the most commonly reported adverse event. Vaccinated cancer patients exhibited a 61% immune response rate, with higher response rates in the breast and ovarian cancer patient cohorts. In keeping with the higher immune efficacy of this vaccine platform, antigen-specific responses were detected in 73% of immune responders after just one vaccination. In 83% of responders, peptide-specific T-cells were detected at two or more time points post-vaccination, with 64% of these patients showing evidence of immune persistence. Immune monitoring also demonstrated the generation of antigen-specific T-cell memory, with the ability to secrete multiple type 1 cytokines. The novel DPX formulation promotes multifunctional effector/memory responses to peptide-based tumor-associated antigens. The data support the capacity of DPX-0907 to elicit type-1 biased immune responses, warranting further clinical development of the vaccine. In this review, we discuss the rationale for developing DPX-based therapeutic cancer vaccine(s), with a focus on DPX-0907, aimed at inducing efficient anti-tumor immunity that may eventually be shown to prolong patient survival.
Keywords: DPX-0907; DepoVax™; cancer vaccine; immunotherapy.
Figures
Similar articles
-
First-in-man application of a novel therapeutic cancer vaccine formulation with the capacity to induce multi-functional T cell responses in ovarian, breast and prostate cancer patients.J Transl Med. 2012 Aug 3;10:156. doi: 10.1186/1479-5876-10-156. J Transl Med. 2012. PMID: 22862954 Free PMC article.
-
A novel breast/ovarian cancer peptide vaccine platform that promotes specific type-1 but not Treg/Tr1-type responses.J Immunother. 2010 Apr;33(3):250-61. doi: 10.1097/CJI.0b013e3181c1f1e9. J Immunother. 2010. PMID: 20445345
-
Type III hypersensitivity reactions to a B cell epitope antigen are abrogated using a depot forming vaccine platform.Hum Vaccin Immunother. 2018 Jan 2;14(1):59-66. doi: 10.1080/21645515.2017.1375637. Epub 2017 Oct 30. Hum Vaccin Immunother. 2018. PMID: 28933663 Free PMC article.
-
Peptide vaccines in breast cancer: The immunological basis for clinical response.Biotechnol Adv. 2015 Dec;33(8):1868-77. doi: 10.1016/j.biotechadv.2015.10.013. Epub 2015 Oct 30. Biotechnol Adv. 2015. PMID: 26523780 Review.
-
Idiotypic vaccination for B-cell malignancies as a model for therapeutic cancer vaccines: from prototype protein to second generation vaccines.Haematologica. 2002 Sep;87(9):989-1001. Haematologica. 2002. PMID: 12217812 Review.
Cited by
-
Engineering Nanoparticles for Targeted Remodeling of the Tumor Microenvironment to Improve Cancer Immunotherapy.Theranostics. 2019 Jan 1;9(1):126-151. doi: 10.7150/thno.29431. eCollection 2019. Theranostics. 2019. PMID: 30662558 Free PMC article. Review.
-
A tumor multicomponent targeting chemoimmune drug delivery system for reprograming the tumor microenvironment and personalized cancer therapy.Drug Discov Today. 2018 Jul;23(7):1344-1356. doi: 10.1016/j.drudis.2018.03.003. Epub 2018 Mar 15. Drug Discov Today. 2018. PMID: 29551455 Free PMC article. Review.
-
Peptide-Based Treatment: A Promising Cancer Therapy.J Immunol Res. 2015;2015:761820. doi: 10.1155/2015/761820. Epub 2015 Oct 19. J Immunol Res. 2015. PMID: 26568964 Free PMC article. Review.
-
Nanotechnology synergized immunoengineering for cancer.Eur J Pharm Biopharm. 2021 Jun;163:72-101. doi: 10.1016/j.ejpb.2021.03.010. Epub 2021 Mar 24. Eur J Pharm Biopharm. 2021. PMID: 33774162 Free PMC article. Review.
-
Breast cancer vaccination comes to age: impacts of bioinformatics.Bioimpacts. 2018;8(3):223-235. doi: 10.15171/bi.2018.25. Epub 2018 Apr 18. Bioimpacts. 2018. PMID: 30211082 Free PMC article. Review.
References
-
- Kantoff PW, Higano CS, Shore ND, et al. IMPACT Study Investigators Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. - PubMed
-
- Perret R, Ronchese F. Memory T cells in cancer immunotherapy: which CD8 T-cell population provides the best protection against tumours? Tissue Antigens. 2008;72(3):187–194. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

