Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota
- PMID: 24591617
- PMCID: PMC3964110
- DOI: 10.1073/pnas.1316569111
Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota
Abstract
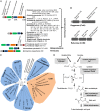
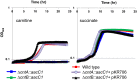
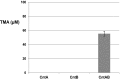
Dietary intake of L-carnitine can promote cardiovascular diseases in humans through microbial production of trimethylamine (TMA) and its subsequent oxidation to trimethylamine N-oxide by hepatic flavin-containing monooxygenases. Although our microbiota are responsible for TMA formation from carnitine, the underpinning molecular and biochemical mechanisms remain unclear. In this study, using bioinformatics approaches, we first identified a two-component Rieske-type oxygenase/reductase (CntAB) and associated gene cluster proposed to be involved in carnitine metabolism in representative genomes of the human microbiota. CntA belongs to a group of previously uncharacterized Rieske-type proteins and has an unusual "bridging" glutamate but not the aspartate residue, which is believed to facilitate intersubunit electron transfer between the Rieske center and the catalytic mononuclear iron center. Using Acinetobacter baumannii as the model, we then demonstrate that cntAB is essential in carnitine degradation to TMA. Heterologous overexpression of cntAB enables Escherichia coli to produce TMA, confirming that these genes are sufficient in TMA formation. Site-directed mutagenesis experiments have confirmed that this unusual "bridging glutamate" residue in CntA is essential in catalysis and neither mutant (E205D, E205A) is able to produce TMA. Taken together, the data in our study reveal the molecular and biochemical mechanisms underpinning carnitine metabolism to TMA in human microbiota and assign the role of this novel group of Rieske-type proteins in microbial carnitine metabolism.
Keywords: comparative genomics; gut microbiota; methylated amine metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Carnitine metabolism in the human gut: characterization of the two-component carnitine monooxygenase CntAB from Acinetobacter baumannii.J Biol Chem. 2020 Sep 11;295(37):13065-13078. doi: 10.1074/jbc.RA120.014266. Epub 2020 Jul 21. J Biol Chem. 2020. PMID: 32694223 Free PMC article.
-
Two-component carnitine monooxygenase from Escherichia coli: functional characterization, inhibition and mutagenesis of the molecular interface.Biosci Rep. 2022 Sep 30;42(9):BSR20221102. doi: 10.1042/BSR20221102. Biosci Rep. 2022. PMID: 36066069 Free PMC article.
-
Light-Activated Electron Transfer and Catalytic Mechanism of Carnitine Oxidation by Rieske-Type Oxygenase from Human Microbiota.Angew Chem Int Ed Engl. 2021 Feb 23;60(9):4529-4534. doi: 10.1002/anie.202012381. Epub 2020 Dec 28. Angew Chem Int Ed Engl. 2021. PMID: 33180358 Free PMC article.
-
Methodological considerations for the identification of choline and carnitine-degrading bacteria in the gut.Methods. 2018 Oct 1;149:42-48. doi: 10.1016/j.ymeth.2018.03.012. Epub 2018 Apr 19. Methods. 2018. PMID: 29684641 Free PMC article. Review.
-
Gut microbiota metabolism of L-carnitine and cardiovascular risk.Atherosclerosis. 2013 Dec;231(2):456-61. doi: 10.1016/j.atherosclerosis.2013.10.013. Epub 2013 Oct 24. Atherosclerosis. 2013. PMID: 24267266 Review.
Cited by
-
Shaping the future of gastrointestinal cancers through metabolic interactions with host gut microbiota.Heliyon. 2024 Jul 27;10(15):e35336. doi: 10.1016/j.heliyon.2024.e35336. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39170494 Free PMC article. Review.
-
Host-microbiome interactions: Gut-Liver axis and its connection with other organs.NPJ Biofilms Microbiomes. 2022 Nov 1;8(1):89. doi: 10.1038/s41522-022-00352-6. NPJ Biofilms Microbiomes. 2022. PMID: 36319663 Free PMC article. Review.
-
Uremic Toxins in the Progression of Chronic Kidney Disease and Cardiovascular Disease: Mechanisms and Therapeutic Targets.Toxins (Basel). 2021 Feb 13;13(2):142. doi: 10.3390/toxins13020142. Toxins (Basel). 2021. PMID: 33668632 Free PMC article. Review.
-
Carnitine in bacterial physiology and metabolism.Microbiology (Reading). 2015 Jun;161(6):1161-74. doi: 10.1099/mic.0.000080. Epub 2015 Mar 18. Microbiology (Reading). 2015. PMID: 25787873 Free PMC article. Review.
-
Role of Trimethylamine N-Oxide in Heart Failure.Rev Cardiovasc Med. 2024 Jul 2;25(7):240. doi: 10.31083/j.rcm2507240. eCollection 2024 Jul. Rev Cardiovasc Med. 2024. PMID: 39139438 Free PMC article. Review.
References
-
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. - PubMed
-
- Turnbaugh PJ, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. - PubMed
-
- Nicholson JK, et al. Host-gut microbiota metabolic interactions. Science. 2012;336(6086):1262–1267. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

