Phosphoregulation of the ceramide transport protein CERT at serine 315 in the interaction with VAMP-associated protein (VAP) for inter-organelle trafficking of ceramide in mammalian cells
- PMID: 24569996
- PMCID: PMC4036191
- DOI: 10.1074/jbc.M113.528380
Phosphoregulation of the ceramide transport protein CERT at serine 315 in the interaction with VAMP-associated protein (VAP) for inter-organelle trafficking of ceramide in mammalian cells
Abstract
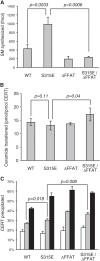
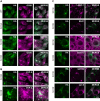
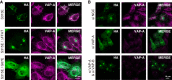
The ceramide transport protein CERT mediates the inter-organelle transport of ceramide for the synthesis of sphingomyelin, presumably through endoplasmic reticulum (ER)-Golgi membrane contact sites. CERT has a short peptide motif named FFAT, which associates with the ER-resident membrane protein VAP. We show that the phosphorylation of CERT at serine 315, which is adjacent to the FFAT motif, markedly enhanced the interaction of CERT with VAP. The phosphomimetic CERT S315E mutant exhibited higher activity to support the ER-to-Golgi transport of ceramide than the wild-type control in a semi-intact cell system, and this enhanced activity was abrogated when its FFAT motif was deleted. The level of phosphorylation of CERT at Ser-315 increased in HeLa cells treated with a sphingolipid biosynthesis inhibitor or exogenous sphingomyelinase. Expression of CERT S315E induced intracellular punctate structures, to which CERT and VAP were co-localized, and the occurrence of the structure was dependent on both phosphatidylinositol 4-monophosphate binding and VAP binding activities of CERT. Phosphorylation of another region (named a serine-rich motif) in CERT is known to down-regulate the activity of CERT. Analysis of various CERT mutant constructs showed that the de-phosphorylation of the serine-rich motif and the phosphorylation of Ser-315 likely have the additive contribution to enhance the activity of CERT. These results demonstrate that the phosphorylation of CERT at the FFAT motif-adjacent serine affected its affinity for VAP, which may regulate the inter-organelle trafficking of ceramide in response to the perturbation of cellular sphingomyelin and/or other sphingolipids.
Keywords: Endoplasmic Reticulum (ER); Golgi; Lipid Transport; Lipid-binding Protein; Sphingolipid.
Figures



Similar articles
-
Efficient trafficking of ceramide from the endoplasmic reticulum to the Golgi apparatus requires a VAMP-associated protein-interacting FFAT motif of CERT.J Biol Chem. 2006 Oct 6;281(40):30279-88. doi: 10.1074/jbc.M605032200. Epub 2006 Aug 8. J Biol Chem. 2006. PMID: 16895911
-
CERT-mediated trafficking of ceramide.Biochim Biophys Acta. 2009 Jul;1791(7):684-91. doi: 10.1016/j.bbalip.2009.01.006. Epub 2009 Jan 22. Biochim Biophys Acta. 2009. PMID: 19416656 Review.
-
CERT and intracellular trafficking of ceramide.Biochim Biophys Acta. 2007 Jun;1771(6):644-53. doi: 10.1016/j.bbalip.2007.01.009. Epub 2007 Jan 23. Biochim Biophys Acta. 2007. PMID: 17314061 Review.
-
Structure, functions and regulation of CERT, a lipid-transfer protein for the delivery of ceramide at the ER-Golgi membrane contact sites.FEBS Lett. 2019 Sep;593(17):2366-2377. doi: 10.1002/1873-3468.13511. Epub 2019 Jul 8. FEBS Lett. 2019. PMID: 31254361 Review.
-
Phosphoinositide binding by the PH domain in ceramide transfer protein (CERT) is inhibited by hyperphosphorylation of an adjacent serine-repeat motif.J Biol Chem. 2018 Jul 13;293(28):11206-11217. doi: 10.1074/jbc.RA118.002465. Epub 2018 May 30. J Biol Chem. 2018. PMID: 29848549 Free PMC article.
Cited by
-
Lipid transfer proteins: the lipid commute via shuttles, bridges and tubes.Nat Rev Mol Cell Biol. 2019 Feb;20(2):85-101. doi: 10.1038/s41580-018-0071-5. Nat Rev Mol Cell Biol. 2019. PMID: 30337668 Review.
-
Hyperosmotic Stress Induces Phosphorylation of CERT and Enhances Its Tethering throughout the Endoplasmic Reticulum.Int J Mol Sci. 2022 Apr 5;23(7):4025. doi: 10.3390/ijms23074025. Int J Mol Sci. 2022. PMID: 35409383 Free PMC article.
-
Systematic prediction of FFAT motifs across eukaryote proteomes identifies nucleolar and eisosome proteins with the predicted capacity to form bridges to the endoplasmic reticulum.Contact (Thousand Oaks). 2019 Jan;2:1-21. doi: 10.1177/2515256419883136. Epub 2019 Oct 30. Contact (Thousand Oaks). 2019. PMID: 31777772 Free PMC article.
-
Developmental regulation of an organelle tether coordinates mitochondrial remodeling in meiosis.J Cell Biol. 2019 Feb 4;218(2):559-579. doi: 10.1083/jcb.201807097. Epub 2018 Dec 11. J Cell Biol. 2019. PMID: 30538140 Free PMC article.
-
Ca2+ and Annexins - Emerging Players for Sensing and Transferring Cholesterol and Phosphoinositides via Membrane Contact Sites.Adv Exp Med Biol. 2023;1422:393-438. doi: 10.1007/978-3-031-21547-6_15. Adv Exp Med Biol. 2023. PMID: 36988890
References
-
- Pewzner-Jung Y., Ben-Dor S., Futerman A. H. (2006) When do Lasses (longevity assurance genes) become CerS (ceramide synthases)? Insights into the regulation of ceramide synthesis. J. Biol. Chem. 281, 25001–25005 - PubMed
-
- Holthuis J. C., Levine T. P. (2005) Lipid traffic: Floppy drives and a superhighway. Nat. Rev. Mol. Cell Biol. 6, 209–220 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

