TRPM2 channels mediate acetaminophen-induced liver damage
- PMID: 24569808
- PMCID: PMC3939869
- DOI: 10.1073/pnas.1322657111
TRPM2 channels mediate acetaminophen-induced liver damage
Abstract
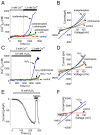
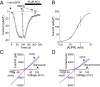
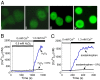
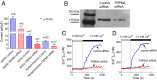
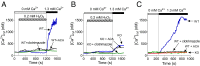
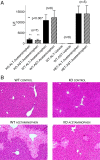
Acetaminophen (paracetamol) is the most frequently used analgesic and antipyretic drug available over the counter. At the same time, acetaminophen overdose is the most common cause of acute liver failure and the leading cause of chronic liver damage requiring liver transplantation in developed countries. Acetaminophen overdose causes a multitude of interrelated biochemical reactions in hepatocytes including the formation of reactive oxygen species, deregulation of Ca(2+) homeostasis, covalent modification and oxidation of proteins, lipid peroxidation, and DNA fragmentation. Although an increase in intracellular Ca(2+) concentration in hepatocytes is a known consequence of acetaminophen overdose, its importance in acetaminophen-induced liver toxicity is not well understood, primarily due to lack of knowledge about the source of the Ca(2+) rise. Here we report that the channel responsible for Ca(2+) entry in hepatocytes in acetaminophen overdose is the Transient Receptor Potential Melanostatine 2 (TRPM2) cation channel. We show by whole-cell patch clamping that treatment of hepatocytes with acetaminophen results in activation of a cation current similar to that activated by H2O2 or the intracellular application of ADP ribose. siRNA-mediated knockdown of TRPM2 in hepatocytes inhibits activation of the current by either acetaminophen or H2O2. In TRPM2 knockout mice, acetaminophen-induced liver damage, assessed by the blood concentration of liver enzymes and liver histology, is significantly diminished compared with wild-type mice. The presented data strongly suggest that TRPM2 channels are essential in the mechanism of acetaminophen-induced hepatocellular death.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Curcumin inhibits activation of TRPM2 channels in rat hepatocytes.Redox Biol. 2016 Apr;7:1-7. doi: 10.1016/j.redox.2015.11.001. Epub 2015 Nov 10. Redox Biol. 2016. PMID: 26609559 Free PMC article.
-
Oxidative stress promotes redistribution of TRPM2 channels to the plasma membrane in hepatocytes.Biochem Biophys Res Commun. 2018 Sep 10;503(3):1891-1896. doi: 10.1016/j.bbrc.2018.07.132. Epub 2018 Jul 31. Biochem Biophys Res Commun. 2018. PMID: 30075844
-
Mechanistic study of TRPM2-Ca(2+)-CAMK2-BECN1 signaling in oxidative stress-induced autophagy inhibition.Autophagy. 2016 Aug 2;12(8):1340-54. doi: 10.1080/15548627.2016.1187365. Epub 2016 May 31. Autophagy. 2016. PMID: 27245989 Free PMC article.
-
TRPM2 Non-Selective Cation Channels in Liver Injury Mediated by Reactive Oxygen Species.Antioxidants (Basel). 2021 Aug 3;10(8):1243. doi: 10.3390/antiox10081243. Antioxidants (Basel). 2021. PMID: 34439491 Free PMC article. Review.
-
TRPM2 cation channels, oxidative stress and neurological diseases: where are we now?Neurochem Res. 2011 Mar;36(3):355-66. doi: 10.1007/s11064-010-0347-4. Epub 2010 Dec 8. Neurochem Res. 2011. PMID: 21140288 Review.
Cited by
-
Circadian Clock Regulation of Hepatic Lipid Metabolism by Modulation of m6A mRNA Methylation.Cell Rep. 2018 Nov 13;25(7):1816-1828.e4. doi: 10.1016/j.celrep.2018.10.068. Cell Rep. 2018. PMID: 30428350 Free PMC article.
-
TRPM2 channel: A novel target for alleviating ischaemia-reperfusion, chronic cerebral hypo-perfusion and neonatal hypoxic-ischaemic brain damage.J Cell Mol Med. 2020 Jan;24(1):4-12. doi: 10.1111/jcmm.14679. Epub 2019 Sep 30. J Cell Mol Med. 2020. PMID: 31568632 Free PMC article. Review.
-
Influence of Acetaminophen on Molecular Adsorption and Transport Properties at Colloidal Liposome Surfaces Studied by Second Harmonic Generation Spectroscopy.Langmuir. 2022 Mar 29;38(12):3852-3859. doi: 10.1021/acs.langmuir.2c00086. Epub 2022 Mar 17. Langmuir. 2022. PMID: 35298170 Free PMC article.
-
TRPM2 protects against cisplatin-induced acute kidney injury and mitochondrial dysfunction via modulating autophagy.Theranostics. 2023 Jul 31;13(13):4356-4375. doi: 10.7150/thno.84655. eCollection 2023. Theranostics. 2023. PMID: 37649595 Free PMC article.
-
Trifluoperazine inhibits acetaminophen-induced hepatotoxicity and hepatic reactive nitrogen formation in mice and in freshly isolated hepatocytes.Toxicol Rep. 2017;4:134-142. doi: 10.1016/j.toxrep.2017.02.005. Toxicol Rep. 2017. PMID: 28503408 Free PMC article.
References
-
- Thomas SHL. Paracetamol (acetaminophen) poisoning. Pharmacol Ther. 1993;60(1):91–120. - PubMed
-
- Mitchell JR, et al. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther. 1973;187(1):185–194. - PubMed
-
- Boyer TD, Rouff SL. Acetaminophen-induced hepatic necrosis and renal failure. JAMA. 1971;218(3):440–441. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

