Kynurenines in CNS disease: regulation by inflammatory cytokines
- PMID: 24567701
- PMCID: PMC3915289
- DOI: 10.3389/fnins.2014.00012
Kynurenines in CNS disease: regulation by inflammatory cytokines
Abstract
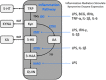
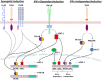
The kynurenine pathway (KP) metabolizes the essential amino acid tryptophan and generates a number of neuroactive metabolites collectively called the kynurenines. Segregated into at least two distinct branches, often termed the "neurotoxic" and "neuroprotective" arms of the KP, they are regulated by the two enzymes kynurenine 3-monooxygenase and kynurenine aminotransferase, respectively. Interestingly, several enzymes in the pathway are under tight control of inflammatory mediators. Recent years have seen a tremendous increase in our understanding of neuroinflammation in CNS disease. This review will focus on the regulation of the KP by inflammatory mediators as it pertains to neurodegenerative and psychiatric disorders.
Keywords: CNS disease; IDO; KAT; KMO; astrocytes; kynurenine; microglia; neuroinflammation.
Figures
Similar articles
-
L-Tryptophan-kynurenine pathway enzymes are therapeutic target for neuropsychiatric diseases: Focus on cell type differences.Neuropharmacology. 2017 Jan;112(Pt B):264-274. doi: 10.1016/j.neuropharm.2016.01.011. Epub 2016 Jan 6. Neuropharmacology. 2017. PMID: 26767951 Review.
-
Interaction of the immune-inflammatory and the kynurenine pathways in rats resistant to antidepressant treatment in model of depression.Int Immunopharmacol. 2019 Aug;73:527-538. doi: 10.1016/j.intimp.2019.05.039. Epub 2019 Jun 5. Int Immunopharmacol. 2019. PMID: 31176083
-
Genetic alterations affecting the genes encoding the enzymes of the kynurenine pathway and their association with human diseases.Mutat Res Rev Mutat Res. 2018 Apr-Jun;776:32-45. doi: 10.1016/j.mrrev.2018.03.001. Epub 2018 Mar 14. Mutat Res Rev Mutat Res. 2018. PMID: 29807576 Review.
-
Neuroactive Kynurenines as Pharmacological Targets: New Experimental Tools and Exciting Therapeutic Opportunities.Pharmacol Rev. 2024 Oct 16;76(6):978-1008. doi: 10.1124/pharmrev.124.000239. Pharmacol Rev. 2024. PMID: 39304346 Review.
-
On the relationship between the two branches of the kynurenine pathway in the rat brain in vivo.J Neurochem. 2009 Apr;109(2):316-25. doi: 10.1111/j.1471-4159.2009.05893.x. Epub 2009 Feb 6. J Neurochem. 2009. PMID: 19226371 Free PMC article.
Cited by
-
Gut-Brain Axis and Neuroinflammation: The Role of Gut Permeability and the Kynurenine Pathway in Neurological Disorders.Cell Mol Neurobiol. 2024 Oct 8;44(1):64. doi: 10.1007/s10571-024-01496-z. Cell Mol Neurobiol. 2024. PMID: 39377830 Free PMC article. Review.
-
The Kynurenine Pathway, Aryl Hydrocarbon Receptor, and Alzheimer's Disease.Brain Sci. 2024 Sep 23;14(9):950. doi: 10.3390/brainsci14090950. Brain Sci. 2024. PMID: 39335444 Free PMC article. Review.
-
Persistent tailoring of MSC activation through genetic priming.Mol Ther Methods Clin Dev. 2024 Aug 8;32(3):101316. doi: 10.1016/j.omtm.2024.101316. eCollection 2024 Sep 12. Mol Ther Methods Clin Dev. 2024. PMID: 39282077 Free PMC article.
-
Does the kynurenine pathway play a pathogenic role in autism spectrum disorder?Brain Behav Immun Health. 2024 Aug 6;40:100839. doi: 10.1016/j.bbih.2024.100839. eCollection 2024 Oct. Brain Behav Immun Health. 2024. PMID: 39263315 Free PMC article.
-
Viral infections in etiology of mental disorders: a broad analysis of cytokine profile similarities - a narrative review.Front Cell Infect Microbiol. 2024 Aug 14;14:1423739. doi: 10.3389/fcimb.2024.1423739. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39206043 Free PMC article. Review.
References
-
- Abbott A., Roberts B. M., Turner L., Campbell D. W., Schaffer C. L., Campbell B., et al. (2010). Inhibition of kynurenine aminotransferase II (KAT II) protects against ketamine-induced cognitive impairment and improves spatial working memory. Soc. Neurosci. Abstr. 472.18
-
- Acuna-Castroviejo D., Tapias V., Lopez L. C., Doerrier C., Camacho E., Carrion M. D., et al. (2011). Protective effects of synthetic kynurenines on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in mice. Brain Res. Bull. 85, 133–140 10.1016/j.brainresbull.2011.03.008 - DOI - PubMed
-
- Akimoto H., Yamada A., Takikawa O. (2007). Up-regulation of the brain indoleamine 2,3-dioxygenase activity in a mouse model of Alzheimer's disease by systemic endotoxin challenge. Int. Cong. 1304, 357–361 10.1016/j.ics.2007.07.026 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

