Identification of Cryptosporidium parvum active chemical series by Repurposing the open access malaria box
- PMID: 24566188
- PMCID: PMC3993250
- DOI: 10.1128/AAC.02641-13
Identification of Cryptosporidium parvum active chemical series by Repurposing the open access malaria box
Abstract
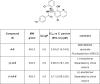
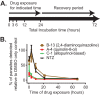
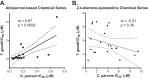
The apicomplexan parasites Cryptosporidium parvum and Cryptosporidium hominis are major etiologic agents of human cryptosporidiosis. The infection is typically self-limited in immunocompetent adults, but it can cause chronic fulminant diarrhea in immunocompromised patients and malnutrition and stunting in children. Nitazoxanide, the current standard of care for cryptosporidiosis, is only partially efficacious for children and is no more effective than a placebo for AIDS patients. Unfortunately, financial obstacles to drug discovery for diseases that disproportionately affect low-income countries and technical limitations associated with studies of Cryptosporidium biology impede the development of better drugs for treating cryptosporidiosis. Using a cell-based high-throughput screen, we queried the Medicines for Malaria Venture (MMV) Open Access Malaria Box for activity against C. parvum. We identified 3 novel chemical series derived from the quinolin-8-ol, allopurinol-based, and 2,4-diamino-quinazoline chemical scaffolds that exhibited submicromolar potency against C. parvum. Potency was conserved in a subset of compounds from each scaffold with varied physicochemical properties, and two of the scaffolds identified exhibit more rapid inhibition of C. parvum growth than nitazoxanide, making them excellent candidates for further development. The 2,4-diamino-quinazoline and allopurinol-based compounds were also potent growth inhibitors of the related apicomplexan parasite Toxoplasma gondii, and a good correlation was observed in the relative activities of the compounds in the allopurinol-based series against T. gondii and C. parvum. Taken together, these data illustrate the utility of the Open Access Malaria Box as a source of both potential leads for drug development and chemical probes to elucidate basic biological processes in C. parvum and other apicomplexan parasites.
Figures

Similar articles
-
A Novel Piperazine-Based Drug Lead for Cryptosporidiosis from the Medicines for Malaria Venture Open-Access Malaria Box.Antimicrob Agents Chemother. 2018 Mar 27;62(4):e01505-17. doi: 10.1128/AAC.01505-17. Print 2018 Apr. Antimicrob Agents Chemother. 2018. PMID: 29339392 Free PMC article.
-
Drug repurposing screen reveals FDA-approved inhibitors of human HMG-CoA reductase and isoprenoid synthesis that block Cryptosporidium parvum growth.Antimicrob Agents Chemother. 2013 Apr;57(4):1804-14. doi: 10.1128/AAC.02460-12. Epub 2013 Feb 4. Antimicrob Agents Chemother. 2013. PMID: 23380723 Free PMC article.
-
A high-throughput phenotypic screen identifies clofazimine as a potential treatment for cryptosporidiosis.PLoS Negl Trop Dis. 2017 Feb 3;11(2):e0005373. doi: 10.1371/journal.pntd.0005373. eCollection 2017 Feb. PLoS Negl Trop Dis. 2017. PMID: 28158186 Free PMC article.
-
Cryptosporidiosis Drug Discovery: Opportunities and Challenges.ACS Infect Dis. 2016 Aug 12;2(8):530-7. doi: 10.1021/acsinfecdis.6b00094. Epub 2016 Jul 22. ACS Infect Dis. 2016. PMID: 27626293 Review.
-
Recent developments in drug discovery against the protozoal parasites Cryptosporidium and Toxoplasma.Bioorg Med Chem Lett. 2017 Apr 1;27(7):1491-1501. doi: 10.1016/j.bmcl.2017.01.046. Epub 2017 Jan 17. Bioorg Med Chem Lett. 2017. PMID: 28242275 Review.
Cited by
-
The ReFRAME library as a comprehensive drug repurposing library and its application to the treatment of cryptosporidiosis.Proc Natl Acad Sci U S A. 2018 Oct 16;115(42):10750-10755. doi: 10.1073/pnas.1810137115. Epub 2018 Oct 3. Proc Natl Acad Sci U S A. 2018. PMID: 30282735 Free PMC article.
-
In Vitro Activities of MMV Malaria Box Compounds against the Apicomplexan Parasite Neospora caninum, the Causative Agent of Neosporosis in Animals.Molecules. 2020 Mar 24;25(6):1460. doi: 10.3390/molecules25061460. Molecules. 2020. PMID: 32213892 Free PMC article.
-
Cryptosporidium spp. diagnosis and research in the 21st century.Food Waterborne Parasitol. 2021 Aug 20;24:e00131. doi: 10.1016/j.fawpar.2021.e00131. eCollection 2021 Sep. Food Waterborne Parasitol. 2021. PMID: 34471706 Free PMC article. Review.
-
Discovery of New Inhibitors of Toxoplasma gondii via the Pathogen Box.Antimicrob Agents Chemother. 2018 Jan 25;62(2):e01640-17. doi: 10.1128/AAC.01640-17. Print 2018 Feb. Antimicrob Agents Chemother. 2018. PMID: 29133550 Free PMC article.
-
Virtual Screening and Experimental Validation Identify Novel Inhibitors of the Plasmodium falciparum Atg8-Atg3 Protein-Protein Interaction.ChemMedChem. 2016 Apr 19;11(8):900-10. doi: 10.1002/cmdc.201500515. Epub 2016 Jan 8. ChemMedChem. 2016. PMID: 26748931 Free PMC article.
References
-
- Yoder JS, Wallace RM, Collier SA, Beach MJ, Hlavsa MC. 2012. Cryptosporidiosis surveillance–United States, 2009–2010. MMWR Morb. Mortal. Wkly. Rep. 61:1–12 - PubMed
-
- Mac Kenzie WR, Hoxie NJ, Proctor ME, Gradus MS, Blair KA, Peterson DE, Kazmierczak JJ, Addiss DG, Fox KR, Rose JB, Davis JP. 1994. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. N. Engl. J. Med. 331:161–167. 10.1056/NEJM199407213310304 - DOI - PubMed
-
- Hlavsa MC, Roberts VA, Anderson AR, Hill VR, Kahler AM, Orr M, Garrison LE, Hicks LA, Newton A, Hilborn ED, Wade TJ, Beach MJ, Yoder JS. 2011. Surveillance for waterborne disease outbreaks and other health events associated with recreational water—United States, 2007–2008. MMWR Morb. Mortal. Wkly. Rep. 60:1–32 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

