Dendritic inhibition in the hippocampus supports fear learning
- PMID: 24558155
- PMCID: PMC4018419
- DOI: 10.1126/science.1247485
Dendritic inhibition in the hippocampus supports fear learning
Abstract
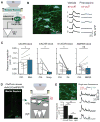
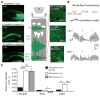
Fear memories guide adaptive behavior in contexts associated with aversive events. The hippocampus forms a neural representation of the context that predicts aversive events. Representations of context incorporate multisensory features of the environment, but must somehow exclude sensory features of the aversive event itself. We investigated this selectivity using cell type-specific imaging and inactivation in hippocampal area CA1 of behaving mice. Aversive stimuli activated CA1 dendrite-targeting interneurons via cholinergic input, leading to inhibition of pyramidal cell distal dendrites receiving aversive sensory excitation from the entorhinal cortex. Inactivating dendrite-targeting interneurons during aversive stimuli increased CA1 pyramidal cell population responses and prevented fear learning. We propose subcortical activation of dendritic inhibition as a mechanism for exclusion of aversive stimuli from hippocampal contextual representations during fear learning.
Figures




Similar articles
-
Synaptic Targeting of Double-Projecting Ventral CA1 Hippocampal Neurons to the Medial Prefrontal Cortex and Basal Amygdala.J Neurosci. 2017 May 10;37(19):4868-4882. doi: 10.1523/JNEUROSCI.3579-16.2017. Epub 2017 Apr 6. J Neurosci. 2017. PMID: 28385873 Free PMC article.
-
Encoding of contextual fear memory in hippocampal-amygdala circuit.Nat Commun. 2020 Mar 13;11(1):1382. doi: 10.1038/s41467-020-15121-2. Nat Commun. 2020. PMID: 32170133 Free PMC article.
-
Aversive stimulus-tuned responses in the CA1 of the dorsal hippocampus.Nat Commun. 2023 Oct 27;14(1):6841. doi: 10.1038/s41467-023-42611-w. Nat Commun. 2023. PMID: 37891171 Free PMC article.
-
The learning of fear extinction.Neurosci Biobehav Rev. 2014 Nov;47:670-83. doi: 10.1016/j.neubiorev.2014.10.016. Neurosci Biobehav Rev. 2014. PMID: 25452113 Review.
-
Neurobiology of Pavlovian fear conditioning.Annu Rev Neurosci. 2001;24:897-931. doi: 10.1146/annurev.neuro.24.1.897. Annu Rev Neurosci. 2001. PMID: 11520922 Review.
Cited by
-
GABA-Synthesizing Enzymes in Calbindin and Calretinin Neurons in Monkey Prefrontal Cortex.Cereb Cortex. 2016 May;26(5):2191-2204. doi: 10.1093/cercor/bhv051. Epub 2015 Mar 30. Cereb Cortex. 2016. PMID: 25824535 Free PMC article.
-
Synergy of direct and indirect cholinergic septo-hippocampal pathways coordinates firing in hippocampal networks.J Neurosci. 2015 Jun 3;35(22):8394-410. doi: 10.1523/JNEUROSCI.4460-14.2015. J Neurosci. 2015. PMID: 26041909 Free PMC article.
-
Parvalbumin and Somatostatin Interneurons Contribute to the Generation of Hippocampal Gamma Oscillations.J Neurosci. 2020 Sep 30;40(40):7668-7687. doi: 10.1523/JNEUROSCI.0261-20.2020. Epub 2020 Aug 28. J Neurosci. 2020. PMID: 32859716 Free PMC article.
-
HIV-1 Tat causes cognitive deficits and selective loss of parvalbumin, somatostatin, and neuronal nitric oxide synthase expressing hippocampal CA1 interneuron subpopulations.J Neurovirol. 2016 Dec;22(6):747-762. doi: 10.1007/s13365-016-0447-2. Epub 2016 May 13. J Neurovirol. 2016. PMID: 27178324 Free PMC article.
-
Adult Hippocampal Neurogenesis, Fear Generalization, and Stress.Neuropsychopharmacology. 2016 Jan;41(1):24-44. doi: 10.1038/npp.2015.167. Epub 2015 Jun 12. Neuropsychopharmacology. 2016. PMID: 26068726 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

