Autonomous parvoviruses neither stimulate nor are inhibited by the type I interferon response in human normal or cancer cells
- PMID: 24554651
- PMCID: PMC3993814
- DOI: 10.1128/JVI.03508-13
Autonomous parvoviruses neither stimulate nor are inhibited by the type I interferon response in human normal or cancer cells
Abstract
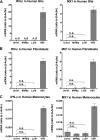
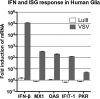
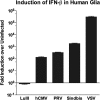
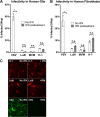
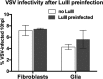
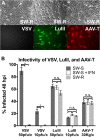
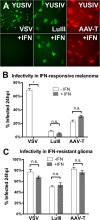
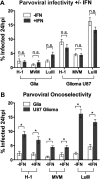
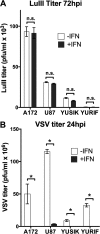
Members of the genus Parvovirus are small, nonenveloped single-stranded DNA viruses that are nonpathogenic in humans but have potential utility as cancer therapeutics. Because the innate immune response to parvoviruses has received relatively little attention, we compared the response to parvoviruses to that of several other types of viruses in human cells. In normal human glia, fibroblasts, or melanocytes, vesicular stomatitis virus evoked robust beta interferon (IFN-β) responses. Cytomegalovirus, pseudorabies virus, and Sindbis virus all evoked a 2-log-unit or greater upregulation of IFN-β in glia; in contrast, LuIII and MVMp parvoviruses did not evoke a detectable IFN-β or interferon-stimulated gene (ISG; MX1, oligoadenylate synthetase [OAS], IFIT-1) response in the same cell types. The lack of response raised the question of whether parvoviral infection can be attenuated by IFN; interestingly, we found that IFN did not decrease parvovirus (MVMp, LuIII, and H-1) infectivity in normal human glia, fibroblasts, or melanocytes. The same was true in human cancers, including glioma, sarcoma, and melanoma. Similarly, IFN failed to attenuate transduction by the dependovirus vector adeno-associated virus type 2. Progeny production of parvoviruses was also unimpaired by IFN in both glioma and melanoma, whereas vesicular stomatitis virus replication was blocked. Sarcoma cells with upregulated IFN signaling that show high levels of resistance to other viruses showed strong infection by LuIII. Unlike many other oncolytic viruses, we found no evidence that impairment of innate immunity in cancer cells plays a role in the oncoselectivity of parvoviruses in human cells. Parvoviral resistance to the effects of IFN in cancer cells may constitute an advantage in the virotherapy of some tumors.
Importance: Understanding the interactions between oncolytic viruses and the innate immune system will facilitate employing these viruses as therapeutic agents in cancer patients. The cancer-selective nature of some oncolytic viruses is based on the impaired innate immunity of many cancer cells. The parvoviruses H-1, LuIII, and MVM target cancer cells; however, their relationship with the innate immune system is relatively uncharacterized. Surprisingly, we found that these parvoviruses do not evoke an interferon response in normal human fibroblasts, glia, or melanocytes. Furthermore, unlike most other types of virus, we found that parvovirus infectivity is unaffected by interferon treatment of human normal or tumor cells. Finally, parvoviral replication was unimpaired by interferon in four human tumor types, including those with residual interferon functionality. We conclude that deficits in the interferon antiviral response of cancer cells do not contribute to parvoviral oncoselectivity in human cells. The interferon-resistant phenotype of parvoviruses may give them an advantage over interferon-sensitive oncolytic viruses in tumors showing residual interferon functionality.
Figures
Similar articles
-
LuIII parvovirus selectively and efficiently targets, replicates in, and kills human glioma cells.J Virol. 2012 Jul;86(13):7280-91. doi: 10.1128/JVI.00227-12. Epub 2012 May 2. J Virol. 2012. PMID: 22553327 Free PMC article.
-
Resistance of pancreatic cancer cells to oncolytic vesicular stomatitis virus: role of type I interferon signaling.Virology. 2013 Feb 5;436(1):221-34. doi: 10.1016/j.virol.2012.11.014. Epub 2012 Dec 14. Virology. 2013. PMID: 23246628 Free PMC article.
-
Inefficient type I interferon-mediated antiviral protection of primary mouse neurons is associated with the lack of apolipoprotein l9 expression.J Virol. 2014 Apr;88(7):3874-84. doi: 10.1128/JVI.03018-13. Epub 2014 Jan 22. J Virol. 2014. PMID: 24453359 Free PMC article.
-
Vectors based on autonomous parvoviruses: novel tools to treat cancer?J Gene Med. 2004 Feb;6 Suppl 1:S193-202. doi: 10.1002/jgm.502. J Gene Med. 2004. PMID: 14978762 Review.
-
Cancer gene therapy through autonomous parvovirus--mediated gene transfer.Curr Gene Ther. 2004 Sep;4(3):249-61. doi: 10.2174/1566523043346228. Curr Gene Ther. 2004. PMID: 15384939 Review.
Cited by
-
Unlocking the promise of oncolytic virotherapy in glioma: combination with chemotherapy to enhance efficacy.Ther Deliv. 2015;6(4):453-68. doi: 10.4155/tde.14.123. Ther Deliv. 2015. PMID: 25996044 Free PMC article. Review.
-
Tumor Selectivity of Oncolytic Parvoviruses: From in vitro and Animal Models to Cancer Patients.Front Bioeng Biotechnol. 2015 Apr 22;3:55. doi: 10.3389/fbioe.2015.00055. eCollection 2015. Front Bioeng Biotechnol. 2015. PMID: 25954743 Free PMC article. Review.
-
Induction of an embryonic mouse innate immune response following inoculation in utero with minute virus of mice.J Virol. 2015 Feb;89(4):2182-91. doi: 10.1128/JVI.02908-14. Epub 2014 Dec 3. J Virol. 2015. PMID: 25473047 Free PMC article.
-
Minute virus of mice shows oncolytic activity against pancreatic cancer cells exhibiting a mesenchymal phenotype.Mol Ther Oncol. 2024 Feb 22;32(1):200780. doi: 10.1016/j.omton.2024.200780. eCollection 2024 Mar 21. Mol Ther Oncol. 2024. PMID: 38596307 Free PMC article.
-
Adenovirus membrane penetration: Tickling the tail of a sleeping dragon.Virology. 2015 May;479-480:591-9. doi: 10.1016/j.virol.2015.03.006. Epub 2015 Mar 19. Virology. 2015. PMID: 25798531 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

