Phase 1 clinical trial of intratumoral reovirus infusion for the treatment of recurrent malignant gliomas in adults
- PMID: 24553100
- PMCID: PMC4015229
- DOI: 10.1038/mt.2014.21
Phase 1 clinical trial of intratumoral reovirus infusion for the treatment of recurrent malignant gliomas in adults
Abstract
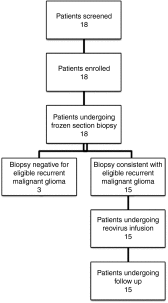
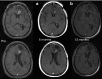
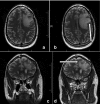
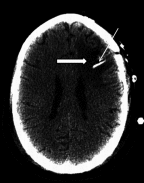
Reovirus, an oncolytic RNA virus exhibiting antiglioma activity, was shown in a previous single institution phase 1 study found that the inoculation of the virus to be well tolerated in patients with recurrent malignant glioma (MG). The goals of multicenter study reported herein were to determine the dose-limiting toxicity, maximum tolerated dose, and target lesion response rate when reovirus was administered in a novel fashion via intratumoral infusion for 72 hours in patients with recurrent malignant glioma. Fifteen adult patients were treated in a dose escalation study ranging from 1 × 10(8) to 1 × 10(10) tissue culture infectious dose 50, tentimes the dose achieved in the previous trial. Neurological, functional examinations, and imaging studies were completed pre- and postinfusion. There was one grade 3 adverse event (convulsions) felt to be possibly related to treatment, but no grade 4 adverse events considered probably or definitely related to treatment. Dose-limiting toxicity were not identified and a maximum tolerated dose was not reached. Evidence of antiglioma activity was seen in some patients. This first report of intratumoral infusion of reovirus in patients with recurrent malignant glioma demonstrated the approach to be safe and well tolerated, warranting further studies.
Figures
Similar articles
-
A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas.Mol Ther. 2008 Mar;16(3):627-32. doi: 10.1038/sj.mt.6300403. Epub 2008 Feb 5. Mol Ther. 2008. PMID: 18253152 Clinical Trial.
-
Non-secreting IL12 expressing oncolytic adenovirus Ad-TD-nsIL12 in recurrent high-grade glioma: a phase I trial.Nat Commun. 2024 Nov 8;15(1):9299. doi: 10.1038/s41467-024-53041-7. Nat Commun. 2024. PMID: 39516192 Free PMC article. Clinical Trial.
-
A phase 1 trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses.Mol Ther. 2014 May;22(5):1048-55. doi: 10.1038/mt.2014.22. Epub 2014 Feb 27. Mol Ther. 2014. PMID: 24572293 Free PMC article. Clinical Trial.
-
[Oncolytic viruses for therapy of malignant glioma].Biomed Khim. 2016 May;62(4):376-90. doi: 10.18097/PBMC20166204376. Biomed Khim. 2016. PMID: 27562991 Review. Russian.
-
Oncolytic herpes simplex virus therapy for malignant glioma: current approaches to successful clinical application.Expert Opin Biol Ther. 2019 Aug;19(8):845-854. doi: 10.1080/14712598.2019.1614557. Epub 2019 May 13. Expert Opin Biol Ther. 2019. PMID: 31046478 Review.
Cited by
-
Investigational new drugs for brain cancer.Expert Opin Investig Drugs. 2016 Aug;25(8):937-56. doi: 10.1080/13543784.2016.1182497. Epub 2016 May 17. Expert Opin Investig Drugs. 2016. PMID: 27170161 Free PMC article. Review.
-
Medical Device Advances in the Treatment of Glioblastoma.Cancers (Basel). 2022 Oct 29;14(21):5341. doi: 10.3390/cancers14215341. Cancers (Basel). 2022. PMID: 36358762 Free PMC article. Review.
-
Review: Oncolytic virotherapy, updates and future directions.Oncotarget. 2017 May 31;8(60):102617-102639. doi: 10.18632/oncotarget.18309. eCollection 2017 Nov 24. Oncotarget. 2017. PMID: 29254276 Free PMC article. Review.
-
Oncolytic Viruses as a Platform for the Treatment of Malignant Brain Tumors.Int J Mol Sci. 2020 Oct 9;21(20):7449. doi: 10.3390/ijms21207449. Int J Mol Sci. 2020. PMID: 33050329 Free PMC article. Review.
-
Intertumoral Differences Dictate the Outcome of TGF-β Blockade on the Efficacy of Viro-Immunotherapy.Cancer Res Commun. 2023 Feb 23;3(2):325-337. doi: 10.1158/2767-9764.CRC-23-0019. eCollection 2023 Feb. Cancer Res Commun. 2023. PMID: 36860656 Free PMC article.
References
-
- Buonerba C, Di Lorenzo G, Marinelli A, Federico P, Palmieri G, Imbimbo M, et al. A comprehensive outlook on intracerebral therapy of malignant gliomas. Crit Rev Oncol Hematol. 2011;80:54–68. - PubMed
-
- Everson RG, Gromeier M, Sampson JH. Viruses in the treatment of malignant glioma. Expert Rev Neurother. 2007;7:321–324. - PubMed
-
- Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–976. - PubMed
-
- Coffey MC, Strong JE, Forsyth PA, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–1334. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

