A charge-inverting mutation in the "linker" region of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors alters agonist binding and gating kinetics independently of allosteric modulators
- PMID: 24550387
- PMCID: PMC4036187
- DOI: 10.1074/jbc.M113.526921
A charge-inverting mutation in the "linker" region of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors alters agonist binding and gating kinetics independently of allosteric modulators
Abstract
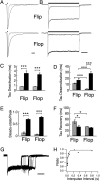
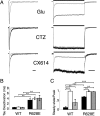
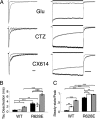
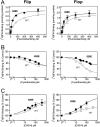
AMPA receptors are gated through binding of glutamate to a solvent-accessible ligand-binding domain. Upon glutamate binding, these receptors undergo a series of conformational rearrangements regulating channel function. Allosteric modulators can bind within a pocket adjacent to the ligand-binding domain to stabilize specific conformations and prevent desensitization. Yelshansky et al. (Yelshansky, M. V., Sobolevsky, A. I., Jatzke, C., and Wollmuth, L. P. (2004) J. Neurosci. 24, 4728-4736) described a model of an electrostatic interaction between the ligand-binding domain and linker region to the pore that regulated channel desensitization. To test this hypothesis, we have conducted a series of experiments focusing on the R628E mutation. Using ultrafast perfusion with voltage clamp, we applied glutamate to outside-out patches pulled from transiently transfected HEK 293 cells expressing wild type or R628E mutant GluA2. In response to a brief pulse of glutamate (1 ms), mutant receptors deactivated with significantly slower kinetics than wild type receptors. In addition, R628E receptors showed significantly more steady-state current in response to a prolonged (500-ms) glutamate application. These changes in receptor kinetics occur through a pathway that is independent of that of allosteric modulators, which show an additive effect on R628E receptors. In addition, ligand binding assays revealed the R628E mutation to have increased affinity for agonist. Finally, we reconciled experimental data with computer simulations that explicitly model mutant and modulator interactions. Our data suggest that R628E stabilizes the receptor closed cleft conformation by reducing agonist dissociation and the transition to the desensitized state. These results suggest that the AMPA receptor external vestibule is a viable target for new positive allosteric modulators.
Keywords: Computer Modeling; Electrophysiology; Gating; Glutamate Receptors, Ionotropic (AMPA, NMDA); Glutamate Receptors, Metabotropic; Structural Biology.
Figures



Similar articles
-
Functional insight into development of positive allosteric modulators of AMPA receptors.Neuropharmacology. 2014 Oct;85:57-66. doi: 10.1016/j.neuropharm.2014.05.022. Epub 2014 May 27. Neuropharmacology. 2014. PMID: 24878241 Free PMC article.
-
Block of AMPA receptor desensitization by a point mutation outside the ligand-binding domain.J Neurosci. 2004 May 19;24(20):4728-36. doi: 10.1523/JNEUROSCI.0757-04.2004. J Neurosci. 2004. PMID: 15152033 Free PMC article.
-
A binding site tyrosine shapes desensitization kinetics and agonist potency at GluR2. A mutagenic, kinetic, and crystallographic study.J Biol Chem. 2005 Oct 21;280(42):35469-76. doi: 10.1074/jbc.M507800200. Epub 2005 Aug 15. J Biol Chem. 2005. PMID: 16103115
-
Pharmacology of AMPA/kainate receptor ligands and their therapeutic potential in neurological and psychiatric disorders.Drugs. 2000 Jan;59(1):33-78. doi: 10.2165/00003495-200059010-00004. Drugs. 2000. PMID: 10718099 Review.
-
[Ligands of the AMPA-subtype glutamate receptors: mechanisms of action and novel chemotypes].Biomed Khim. 2021 May;67(3):187-200. doi: 10.18097/PBMC20216703187. Biomed Khim. 2021. PMID: 34142526 Review. Russian.
Cited by
-
Identification of critical functional determinants of kainate receptor modulation by auxiliary protein Neto2.J Physiol. 2015 Nov 15;593(22):4815-33. doi: 10.1113/JP271103. Epub 2015 Sep 20. J Physiol. 2015. PMID: 26282342 Free PMC article.
-
Two adjacent phenylalanines in the NMDA receptor GluN2A subunit M3 domain interactively regulate alcohol sensitivity and ion channel gating.Neuropharmacology. 2017 Mar 1;114:20-33. doi: 10.1016/j.neuropharm.2016.11.013. Epub 2016 Nov 19. Neuropharmacology. 2017. PMID: 27876530 Free PMC article.
-
Unitary Properties of AMPA Receptors with Reduced Desensitization.Biophys J. 2017 Nov 21;113(10):2218-2235. doi: 10.1016/j.bpj.2017.07.030. Epub 2017 Aug 30. Biophys J. 2017. PMID: 28863863 Free PMC article.
-
The quantal component of synaptic transmission from sensory hair cells to the vestibular calyx.J Neurophysiol. 2015 Jun 1;113(10):3827-35. doi: 10.1152/jn.00055.2015. Epub 2015 Apr 15. J Neurophysiol. 2015. PMID: 25878150 Free PMC article.
References
-
- Partin K. M., Mayer M. L. (1996) Negative allosteric modulation of wild-type and mutants AMPA receptors by GYKI 53655. Mol. Pharmacol. 49, 142–148 - PubMed
-
- Armstrong N., Gouaux E. (2000) Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron 28, 165–181 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

