MicroRNA-like viral small RNA from Dengue virus 2 autoregulates its replication in mosquito cells
- PMID: 24550303
- PMCID: PMC3932895
- DOI: 10.1073/pnas.1320123111
MicroRNA-like viral small RNA from Dengue virus 2 autoregulates its replication in mosquito cells
Abstract
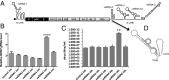
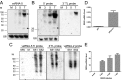
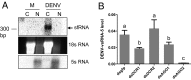
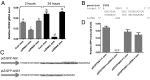
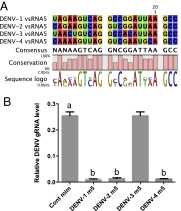
MicroRNAs (miRNAs) are small regulatory RNAs that play significant roles in most cellular processes. In the seemingly endless arms race between hosts and pathogens, viruses also encode miRNAs that facilitate successful infection. In search of functional miRNAs or viral small RNAs (vsRNAs) encoded by Dengue virus (DENV), deep sequencing data of virus-infected Aedes aegypti mosquitoes were used. From six vsRNAs, with candidate stem-loop structures in the 5' and 3' untranslated regions of the viral genomic RNA, inhibition of DENV-vsRNA-5 led to significant increases in viral replication. Silencing of RNA interference (RNAi)/miRNA pathways' associated proteins showed that Argonaute 2 is mainly involved in DENV-vsRNA-5 biogenesis. Cloning of the precursor stem loop, immunoprecipitations, ectopic expression and detection in RNAi-deficient C6/36, and the mammalian Vero cell lines further confirmed DENV-vsRNA-5 production. Furthermore, significant impact of synthetic mimic and inhibitor of DENV-vsRNA-5 on DENV RNA levels revealed DENV-vsRNA-5's role in virus autoregulation by targeting the virus nonstructural protein 1 gene. Notably, DENV-vsRNA-5 homologous mimics from DENV serotypes 1 and 4, but not 3, inhibited DENV-2 replication. The results revealed that DENV is able to encode functional vsRNAs, and one of those, which resembles miRNAs, specifically targets a viral gene, opening an avenue for possible utilization of the small RNA to limit DENV replication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Comment in
-
A "microRNA-like" small RNA expressed by Dengue virus?Proc Natl Acad Sci U S A. 2014 Jun 10;111(23):E2359. doi: 10.1073/pnas.1406854111. Epub 2014 May 22. Proc Natl Acad Sci U S A. 2014. PMID: 24853506 Free PMC article. No abstract available.
-
Are viral small RNA regulating Dengue virus replication beyond serotype 2?Proc Natl Acad Sci U S A. 2014 Jul 22;111(29):E2915-6. doi: 10.1073/pnas.1409972111. Epub 2014 Jul 14. Proc Natl Acad Sci U S A. 2014. PMID: 25024232 Free PMC article. No abstract available.
-
Reply to Skalsky et al.: A microRNA-like small RNA from Dengue virus.Proc Natl Acad Sci U S A. 2014 Jun 10;111(23):E2360. doi: 10.1073/pnas.1407176111. Proc Natl Acad Sci U S A. 2014. PMID: 25061641 Free PMC article. No abstract available.
-
Reply to Finol: Viral small RNA from Dengue virus and its regulatory role in different serotypes.Proc Natl Acad Sci U S A. 2014 Jul 22;111(29):E2917. doi: 10.1073/pnas.1410765111. Proc Natl Acad Sci U S A. 2014. PMID: 25187918 Free PMC article. No abstract available.
Similar articles
-
Small RNA Profiling in Dengue Virus 2-Infected Aedes Mosquito Cells Reveals Viral piRNAs and Novel Host miRNAs.PLoS Negl Trop Dis. 2016 Feb 25;10(2):e0004452. doi: 10.1371/journal.pntd.0004452. eCollection 2016 Feb. PLoS Negl Trop Dis. 2016. PMID: 26914027 Free PMC article.
-
The Involvement of Atlastin in Dengue Virus and Wolbachia Infection in Aedes aegypti and Its Regulation by aae-miR-989.Microbiol Spectr. 2022 Oct 26;10(5):e0225822. doi: 10.1128/spectrum.02258-22. Epub 2022 Sep 27. Microbiol Spectr. 2022. PMID: 36165808 Free PMC article.
-
Screening for differentially expressed miRNAs in Aedes albopictus (Diptera: Culicidae) exposed to DENV-2 and their effect on replication of DENV-2 in C6/36 cells.Parasit Vectors. 2019 Jan 18;12(1):44. doi: 10.1186/s13071-018-3261-2. Parasit Vectors. 2019. PMID: 30658692 Free PMC article.
-
Cross Talk between MicroRNAs and Dengue Virus.Am J Trop Med Hyg. 2024 Apr 2;110(5):856-867. doi: 10.4269/ajtmh.23-0546. Print 2024 May 1. Am J Trop Med Hyg. 2024. PMID: 38579704 Review.
-
Host and viral non-coding RNAs in dengue pathogenesis.Rev Med Virol. 2022 Nov;32(6):e2360. doi: 10.1002/rmv.2360. Epub 2022 May 5. Rev Med Virol. 2022. PMID: 35510480 Review.
Cited by
-
Differential miRNA Expression Profiling Reveals Correlation of miR125b-5p with Persistent Infection of Japanese Encephalitis Virus.Int J Mol Sci. 2021 Apr 19;22(8):4218. doi: 10.3390/ijms22084218. Int J Mol Sci. 2021. PMID: 33921710 Free PMC article.
-
Profile of Small RNAs, vDNA Forms and Viral Integrations in Late Chikungunya Virus Infection of Aedes albopictus Mosquitoes.Viruses. 2021 Mar 25;13(4):553. doi: 10.3390/v13040553. Viruses. 2021. PMID: 33806250 Free PMC article.
-
RNA Virus-Encoded miRNAs: Current Insights and Future Challenges.Front Microbiol. 2021 Jun 24;12:679210. doi: 10.3389/fmicb.2021.679210. eCollection 2021. Front Microbiol. 2021. PMID: 34248890 Free PMC article. Review.
-
Small RNA Profiling in Dengue Virus 2-Infected Aedes Mosquito Cells Reveals Viral piRNAs and Novel Host miRNAs.PLoS Negl Trop Dis. 2016 Feb 25;10(2):e0004452. doi: 10.1371/journal.pntd.0004452. eCollection 2016 Feb. PLoS Negl Trop Dis. 2016. PMID: 26914027 Free PMC article.
-
Standing your ground to exoribonucleases: Function of Flavivirus long non-coding RNAs.Virus Res. 2016 Jan 2;212:70-7. doi: 10.1016/j.virusres.2015.09.009. Epub 2015 Sep 11. Virus Res. 2016. PMID: 26368052 Free PMC article. Review.
References
-
- Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: The spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10(12) Suppl:S98–S109. - PubMed
-
- Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10(2):100–103. - PubMed
-
- Scott TW, Morrison AC. Vector dynamics and transmission of dengue virus: Implications for dengue surveillance and prevention strategies: Vector dynamics and dengue prevention. Curr Top Microbiol Immunol. 2010;338:115–128. - PubMed
-
- Alvarez DE, De Lella Ezcurra AL, Fucito S, Gamarnik AV. Role of RNA structures present at the 3’UTR of dengue virus on translation, RNA synthesis, and viral replication. Virology. 2005;339(2):200–212. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

