Life of psi: how full-length HIV-1 RNAs become packaged genomes in the viral particles
- PMID: 24530126
- PMCID: PMC6258065
- DOI: 10.1016/j.virol.2014.01.019
Life of psi: how full-length HIV-1 RNAs become packaged genomes in the viral particles
Abstract
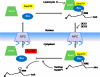
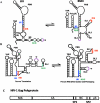
As a member of the retrovirus family, HIV-1 packages its RNA genome into particles and replicates through a DNA intermediate that integrates into the host cellular genome. The multiple genes encoded by HIV-1 are expressed from the same promoter and their expression is regulated by splicing and ribosomal frameshift. The full-length HIV-1 RNA plays a central role in viral replication as it serves as the genome in the progeny virus and is used as the template for Gag and GagPol translation. In this review, we summarize findings that contribute to our current understanding of how full-length RNA is expressed and transported, cis- and trans-acting elements important for RNA packaging, the locations and timing of RNA:RNA and RNA:Gag interactions, and the processes required for this RNA to be packaged into viral particles.
Keywords: Full-length RNA; Gag; HIV-1; RNA dimerization; RNA export; RNA packaging; RNA transcription and processing; RRE; Retrovirus; Rev; Virus assembly.
Published by Elsevier Inc.
Figures

Similar articles
-
Subcellular Localization of HIV-1 gag-pol mRNAs Regulates Sites of Virion Assembly.J Virol. 2017 Feb 28;91(6):e02315-16. doi: 10.1128/JVI.02315-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28053097 Free PMC article.
-
Interactions between HIV-1 Gag and Viral RNA Genome Enhance Virion Assembly.J Virol. 2017 Jul 27;91(16):e02319-16. doi: 10.1128/JVI.02319-16. Print 2017 Aug 15. J Virol. 2017. PMID: 28539452 Free PMC article.
-
The HIV-1 Rev/RRE system is required for HIV-1 5' UTR cis elements to augment encapsidation of heterologous RNA into HIV-1 viral particles.Retrovirology. 2011 Jun 24;8:51. doi: 10.1186/1742-4690-8-51. Retrovirology. 2011. PMID: 21702950 Free PMC article.
-
HIV-1 RNA genome packaging: it's G-rated.mBio. 2024 Apr 10;15(4):e0086123. doi: 10.1128/mbio.00861-23. Epub 2024 Feb 27. mBio. 2024. PMID: 38411060 Free PMC article. Review.
-
RNA Packaging in HIV.Trends Microbiol. 2019 Aug;27(8):715-723. doi: 10.1016/j.tim.2019.04.003. Epub 2019 May 10. Trends Microbiol. 2019. PMID: 31085095 Free PMC article. Review.
Cited by
-
From Cells to Virus Particles: Quantitative Methods to Monitor RNA Packaging.Viruses. 2016 Aug 22;8(8):239. doi: 10.3390/v8080239. Viruses. 2016. PMID: 27556480 Free PMC article. Review.
-
NMR Studies of Retroviral Genome Packaging.Viruses. 2020 Sep 30;12(10):1115. doi: 10.3390/v12101115. Viruses. 2020. PMID: 33008123 Free PMC article. Review.
-
Exploring HIV-1 Maturation: A New Frontier in Antiviral Development.Viruses. 2024 Sep 6;16(9):1423. doi: 10.3390/v16091423. Viruses. 2024. PMID: 39339899 Free PMC article. Review.
-
HIV RGB: Automated Single-Cell Analysis of HIV-1 Rev-Dependent RNA Nuclear Export and Translation Using Image Processing in KNIME.Viruses. 2022 Apr 26;14(5):903. doi: 10.3390/v14050903. Viruses. 2022. PMID: 35632645 Free PMC article.
-
HIV-1 Packaging Visualised by In-Gel SHAPE.Viruses. 2021 Nov 29;13(12):2389. doi: 10.3390/v13122389. Viruses. 2021. PMID: 34960658 Free PMC article.
References
-
- Abbink TEM, Berkhout B. A novel long distance base-pairing interaction in human immunodeficiency virus type 1 RNA occludes the Gag start codon. J. Biol. Chem. 2003;278:11601–11611. - PubMed
-
- Adams M, Sharmeen L, Kimpton J, Romeo JM, Garcia JV, Peterlin BM, Groudine M, Emerman M. Cellular latency in human immunodeficiency virus-infected individuals with high CD4 levels can be detected by the presence of promoter-proximal transcripts. Proc. Natl. Acad. Sci. U. S. A. 1994;91:3862–3866. - PMC - PubMed
-
- Awang G, Sen D. Mode of dimerization of HIV-1 genomic RNA. Biochemistry (Mosc.) 1993;32:11453–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

