C. elegans ciliated sensory neurons release extracellular vesicles that function in animal communication
- PMID: 24530063
- PMCID: PMC4659354
- DOI: 10.1016/j.cub.2014.01.002
C. elegans ciliated sensory neurons release extracellular vesicles that function in animal communication
Abstract
Cells release extracellular vesicles (ECVs) that play important roles in intercellular communication and may mediate a broad range of physiological and pathological processes. Many fundamental aspects of ECV biogenesis and signaling have yet to be determined, with ECV detection being a challenge and obstacle due to the small size (100 nm) of the ECVs. We developed an in vivo system to visualize the dynamic release of GFP-labeled ECVs. We show here that specific Caenorhabdidits elegans ciliated sensory neurons shed and release ECVs containing GFP-tagged polycystins LOV-1 and PKD-2. These ECVs are also abundant in the lumen surrounding the cilium. Electron tomography and genetic analysis indicate that ECV biogenesis occurs via budding from the plasma membrane at the ciliary base and not via fusion of multivesicular bodies. Intraflagellar transport and kinesin-3 KLP-6 are required for environmental release of PKD-2::GFP-containing ECVs. ECVs isolated from wild-type animals induce male tail-chasing behavior, while ECVs isolated from klp-6 animals and lacking PKD-2::GFP do not. We conclude that environmentally released ECVs play a role in animal communication and mating-related behaviors.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

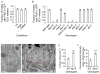
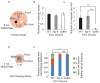
Similar articles
-
Ciliary intrinsic mechanisms regulate dynamic ciliary extracellular vesicle release from sensory neurons.Curr Biol. 2024 Jun 17;34(12):2756-2763.e2. doi: 10.1016/j.cub.2024.05.015. Epub 2024 Jun 4. Curr Biol. 2024. PMID: 38838665
-
The KLP-6 kinesin is required for male mating behaviors and polycystin localization in Caenorhabditis elegans.Curr Biol. 2005 Mar 8;15(5):394-404. doi: 10.1016/j.cub.2004.12.073. Curr Biol. 2005. PMID: 15753033
-
Mating behavior, male sensory cilia, and polycystins in Caenorhabditis elegans.Semin Cell Dev Biol. 2014 Sep;33:25-33. doi: 10.1016/j.semcdb.2014.06.001. Epub 2014 Jun 27. Semin Cell Dev Biol. 2014. PMID: 24977333 Free PMC article. Review.
-
The tubulin deglutamylase CCPP-1 regulates the function and stability of sensory cilia in C. elegans.Curr Biol. 2011 Oct 25;21(20):1685-94. doi: 10.1016/j.cub.2011.08.049. Epub 2011 Oct 6. Curr Biol. 2011. PMID: 21982591 Free PMC article.
-
Mating worms and the cystic kidney: Caenorhabditis elegans as a model for renal disease.Pediatr Nephrol. 2005 Nov;20(11):1531-6. doi: 10.1007/s00467-005-1958-x. Epub 2005 Jun 10. Pediatr Nephrol. 2005. PMID: 15947985 Review.
Cited by
-
Cell type-specific structural plasticity of the ciliary transition zone in C. elegans.Biol Cell. 2019 Apr;111(4):95-107. doi: 10.1111/boc.201800042. Epub 2019 Feb 14. Biol Cell. 2019. PMID: 30681171 Free PMC article.
-
Sexually Dimorphic Regulation of Behavioral States by Dopamine in Caenorhabditis elegans.J Neurosci. 2019 Jun 12;39(24):4668-4683. doi: 10.1523/JNEUROSCI.2985-18.2019. Epub 2019 Apr 15. J Neurosci. 2019. PMID: 30988167 Free PMC article.
-
Emerging roles of extracellular vesicles in the nervous system.J Neurosci. 2014 Nov 12;34(46):15482-9. doi: 10.1523/JNEUROSCI.3258-14.2014. J Neurosci. 2014. PMID: 25392515 Free PMC article. Review.
-
The mini player with diverse functions: extracellular vesicles in cell biology, disease, and therapeutics.Protein Cell. 2022 Sep;13(9):631-654. doi: 10.1007/s13238-021-00863-6. Epub 2021 Aug 10. Protein Cell. 2022. PMID: 34374936 Free PMC article. Review.
-
Cell-Specific α-Tubulin Isotype Regulates Ciliary Microtubule Ultrastructure, Intraflagellar Transport, and Extracellular Vesicle Biology.Curr Biol. 2017 Apr 3;27(7):968-980. doi: 10.1016/j.cub.2017.02.039. Epub 2017 Mar 16. Curr Biol. 2017. PMID: 28318980 Free PMC article.
References
-
- Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol. 1986;117:456–487. - PubMed
-
- Sulston JE, Albertson DG, Thomson JN. The Caenorhabditis elegans male: postembryonic development of nongonadal structures. Dev Biol. 1980;78:542–576. - PubMed
-
- Ward S, Thomson N, White JG, Brenner S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J Comp Neurol. 1975;160:313–337. - PubMed
-
- Barr MM, DeModena J, Braun D, Nguyen CQ, Hall DH, Sternberg PW. The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Curr Biol. 2001;11:1341–1346. - PubMed
-
- Barr MM, Sternberg PW. A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature. 1999;401:386–389. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

