Two regions of the ryanodine receptor calcium channel are involved in Ca(2+)-dependent inactivation
- PMID: 24521037
- PMCID: PMC3985739
- DOI: 10.1021/bi401586h
Two regions of the ryanodine receptor calcium channel are involved in Ca(2+)-dependent inactivation
Abstract
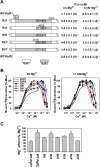
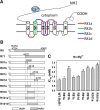
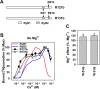
Skeletal (RyR1) and cardiac muscle (RyR2) isoforms of ryanodine receptor calcium channels are inhibited by millimollar Ca(2+), but the affinity of RyR2 for inhibitory Ca(2+) is ~10 times lower than that of RyR1. Previous studies demonstrated that the C-terminal quarter of RyR has critical domain(s) for Ca(2+) inactivation. To obtain further insights into the molecular basis of regulation of RyRs by Ca(2+), we constructed and expressed 18 RyR1-RyR2 chimeras in HEK293 cells and determined the Ca(2+) activation and inactivation affinities of these channels using the [(3)H]ryanodine binding assay. Replacing two distinct regions of RyR1 with corresponding RyR2 sequences reduced the affinity for Ca(2+) inactivation. The first region (RyR2 amino acids 4020-4250) contains two EF-hand Ca(2+) binding motifs (EF1, amino acids 4036-4047; EF2, amino acids 4071-4082), and the second region includes the putative second transmembrane segment (S2). A RyR1-backbone chimera containing only EF2 from RyR2 had a modest (not significant) change in Ca(2+) inactivation, whereas another chimera channel carrying only EF1 from RyR2 had a significantly reduced level of Ca(2+) inactivation. The results suggest that EF1 is a more critical determinant for RyR inactivation by Ca(2+). In addition, activities of the chimera carrying RyR2 EF-hands were suppressed at 10-100 μM Ca(2+), and the suppression was relieved by 1 mM Mg(2+). The same effects have been observed with wild-type RyR2. A mutant RyR1 carrying both regions replaced with RyR2 sequences (amino acids 4020-4250 and 4560-4618) showed a Ca(2+) inactivation affinity comparable to that of RyR2, indicating that these regions are sufficient to confer RyR2-type Ca(2+)-dependent inactivation on RyR1.
Figures



Similar articles
-
Two EF-hand motifs in ryanodine receptor calcium release channels contribute to isoform-specific regulation by calmodulin.Cell Calcium. 2017 Sep;66:62-70. doi: 10.1016/j.ceca.2017.05.013. Epub 2017 Jun 6. Cell Calcium. 2017. PMID: 28807150 Free PMC article.
-
Ca(2+) inactivation sites are located in the COOH-terminal quarter of recombinant rabbit skeletal muscle Ca(2+) release channels (ryanodine receptors).J Biol Chem. 1999 Sep 10;274(37):26120-6. doi: 10.1074/jbc.274.37.26120. J Biol Chem. 1999. PMID: 10473562
-
Malignant hyperthermia-associated mutations in the S2-S3 cytoplasmic loop of type 1 ryanodine receptor calcium channel impair calcium-dependent inactivation.Am J Physiol Cell Physiol. 2016 Nov 1;311(5):C749-C757. doi: 10.1152/ajpcell.00134.2016. Epub 2016 Aug 24. Am J Physiol Cell Physiol. 2016. PMID: 27558158 Free PMC article.
-
Insight towards the identification of cytosolic Ca2+ -binding sites in ryanodine receptors from skeletal and cardiac muscle.Acta Physiol (Oxf). 2017 Apr;219(4):757-767. doi: 10.1111/apha.12772. Epub 2016 Sep 27. Acta Physiol (Oxf). 2017. PMID: 27543850 Review.
-
Molecular Insights into Calcium Dependent Regulation of Ryanodine Receptor Calcium Release Channels.Adv Exp Med Biol. 2020;1131:321-336. doi: 10.1007/978-3-030-12457-1_13. Adv Exp Med Biol. 2020. PMID: 31646516 Review.
Cited by
-
Extensive Ca2+ leak through K4750Q cardiac ryanodine receptors caused by cytosolic and luminal Ca2+ hypersensitivity.J Gen Physiol. 2017 Feb;149(2):199-218. doi: 10.1085/jgp.201611624. Epub 2017 Jan 12. J Gen Physiol. 2017. PMID: 28082361 Free PMC article.
-
Structural Insight Into Ryanodine Receptor Channelopathies.Front Pharmacol. 2022 May 23;13:897494. doi: 10.3389/fphar.2022.897494. eCollection 2022. Front Pharmacol. 2022. PMID: 35677449 Free PMC article. Review.
-
The EF-hand Ca2+ Binding Domain Is Not Required for Cytosolic Ca2+ Activation of the Cardiac Ryanodine Receptor.J Biol Chem. 2016 Jan 29;291(5):2150-60. doi: 10.1074/jbc.M115.693325. Epub 2015 Dec 9. J Biol Chem. 2016. PMID: 26663082 Free PMC article.
-
Ryanodine Receptor Structure and Function in Health and Disease.Subcell Biochem. 2018;87:329-352. doi: 10.1007/978-981-10-7757-9_11. Subcell Biochem. 2018. PMID: 29464565 Free PMC article. Review.
-
Lanthanides Report Calcium Sensor in the Vestibule of Ryanodine Receptor.Biophys J. 2017 May 23;112(10):2127-2137. doi: 10.1016/j.bpj.2017.03.023. Biophys J. 2017. PMID: 28538150 Free PMC article.
References
-
- Franzini-Armstrong C.; Protasi F. (1997) Ryanodine receptors of striated muscles: A complex channel capable of multiple interactions. Physiol. Rev. 77, 699–729. - PubMed
-
- Meissner G. (2002) Regulation of mammalian ryanodine receptors. Front. Biosci. 7, d2072–d2080. - PubMed
-
- Chen S. R. W.; Maclennan D. H. (1994) Identification of calmodulin-, Ca2+-, and ruthenium red-binding domains in the Ca2+ release channel (ryanodine receptor) of rabbit skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 269, 22698–22704. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

