Intracellular trafficking of the human cytomegalovirus-encoded 7-trans-membrane protein homologs pUS27 and pUL78 during viral infection: a comparative analysis
- PMID: 24517969
- PMCID: PMC3939477
- DOI: 10.3390/v6020661
Intracellular trafficking of the human cytomegalovirus-encoded 7-trans-membrane protein homologs pUS27 and pUL78 during viral infection: a comparative analysis
Abstract
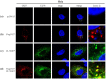
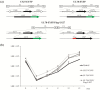
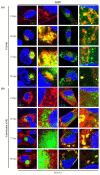
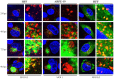
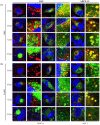
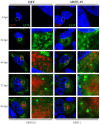
Human cytomegalovirus (HCMV) encodes four G protein-coupled receptor (GPCR) homologs, termed pUS27, pUS28, pUL33, and pUL78. In contrast to the extensively characterized vGPCRs pUS28 and pUL33, knowledge concerning pUS27 and pUL78 is limited. Previous studies already demonstrated constitutive internalization of pUS27 and pUL78, as well as an association with the endosomal machinery, however, these results were mainly obtained using transiently transfected cells. To explore the subcellular localization of both receptors during viral infection, we constructed recombinant HCMVs expressing tagged vGPCRs. Colocalization analyses revealed a predominant association of pUS27 or pUL78 with the trans-Golgi network or the endoplasmic reticulum, respectively. Intriguingly, our data emphasize that protein sorting is highly regulated by viral functions as we detected dramatic changes in the colocalization of pUS27 and pUL78 with endosomal markers during progression of HCMV replication. Furthermore, we observed cell type-dependent differences in trafficking of both vGPCRs between fibroblasts and epithelial cells. Most importantly, infection experiments with a recombinant HCMV carrying tagged versions of pUS27 and pUL78 simultaneously, revealed that these two proteins do not colocalize during viral infection. This contrasts to results of transient expression experiments. In conclusion, our results highlight the importance to investigate vGPCR trafficking in a viral context.
Figures

Similar articles
-
Emerging roles of cytomegalovirus-encoded G protein-coupled receptors during lytic and latent infection.Med Microbiol Immunol. 2019 Aug;208(3-4):447-456. doi: 10.1007/s00430-019-00595-9. Epub 2019 Mar 21. Med Microbiol Immunol. 2019. PMID: 30900091 Review.
-
Human cytomegalovirus pUS27 G protein-coupled receptor homologue is required for efficient spread by the extracellular route but not for direct cell-to-cell spread.J Virol. 2011 Apr;85(8):3700-7. doi: 10.1128/JVI.02442-10. Epub 2011 Feb 9. J Virol. 2011. PMID: 21307184 Free PMC article.
-
Human cytomegalovirus pUL78 G protein-coupled receptor homologue is required for timely cell entry in epithelial cells but not fibroblasts.J Virol. 2012 Nov;86(21):11425-33. doi: 10.1128/JVI.05900-11. Epub 2012 Aug 22. J Virol. 2012. PMID: 22915800 Free PMC article.
-
The 7-transmembrane protein homologue UL78 of the human cytomegalovirus forms oligomers and traffics between the plasma membrane and different intracellular compartments.Arch Virol. 2012 May;157(5):935-49. doi: 10.1007/s00705-012-1246-6. Epub 2012 Feb 12. Arch Virol. 2012. PMID: 22327422
-
HCMV-encoded G-protein-coupled receptors as constitutively active modulators of cellular signaling networks.Trends Pharmacol Sci. 2006 Jan;27(1):56-63. doi: 10.1016/j.tips.2005.11.006. Epub 2005 Dec 13. Trends Pharmacol Sci. 2006. PMID: 16352349 Review.
Cited by
-
Inferring differential subcellular localisation in comparative spatial proteomics using BANDLE.Nat Commun. 2022 Oct 10;13(1):5948. doi: 10.1038/s41467-022-33570-9. Nat Commun. 2022. PMID: 36216816 Free PMC article.
-
Emerging roles of cytomegalovirus-encoded G protein-coupled receptors during lytic and latent infection.Med Microbiol Immunol. 2019 Aug;208(3-4):447-456. doi: 10.1007/s00430-019-00595-9. Epub 2019 Mar 21. Med Microbiol Immunol. 2019. PMID: 30900091 Review.
-
The human cytomegalovirus chemokine receptor homolog encoded by US27.Virus Genes. 2017 Aug;53(4):516-521. doi: 10.1007/s11262-017-1462-y. Epub 2017 Apr 26. Virus Genes. 2017. PMID: 28447191 Review.
-
Attenuation of chemokine receptor function and surface expression as an immunomodulatory strategy employed by human cytomegalovirus is linked to vGPCR US28.Cell Commun Signal. 2016 Dec 12;14(1):31. doi: 10.1186/s12964-016-0154-x. Cell Commun Signal. 2016. PMID: 27955674 Free PMC article.
-
Modulation of cellular signaling by herpesvirus-encoded G protein-coupled receptors.Front Pharmacol. 2015 Mar 9;6:40. doi: 10.3389/fphar.2015.00040. eCollection 2015. Front Pharmacol. 2015. PMID: 25805993 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

