Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH
- PMID: 24516018
- PMCID: PMC3940537
- DOI: 10.1200/JCO.2013.49.3726
Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH
Abstract
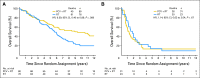
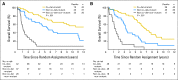
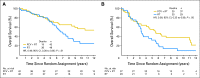
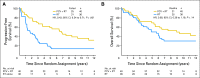
Purpose: Patients with 1p/19q codeleted anaplastic oligodendroglial tumors who participated in RTOG (Radiation Therapy Oncology Group) 9402 lived much longer after chemoradiotherapy (CRT) than radiation therapy (RT) alone. However, some patients with noncodeleted tumors also benefited from CRT; survival curves separated after the median had been reached, and significantly more patients lived ≥ 10 years after CRT than RT. Thus, 1p/19q status may not identify all responders to CRT.
Patients and methods: Using trial data, we inquired whether an IDH mutation or germ-line polymorphism associated with IDH-mutant gliomas identified the patients in RTOG 9402 who benefited from CRT.
Results: IDH status was evaluable in 210 of 291 patients; 156 (74%) had mutations. rs55705857 was evaluable in 245 patients; 76 (31%) carried the G risk allele. Both were associated with longer progression-free survival after CRT, and mutant IDH was associated with longer overall survival (9.4 v 5.7 years; hazard ratio [HR], 0.59; 95% CI, 0.40 to 0.86; P = .006). For those with wild-type tumors, CRT did not prolong median survival (1.3 v 1.8 years; HR, 1.14; 95% CI, 0.63 to 2.04; P = .67) or 10-year survival rate (CRT, 6% v RT, 4%). Patients with codeleted mutated tumors (14.7 v 6.8 years; HR, 0.49; 95% CI, 0.28 to 0.85; P = .01) and noncodeleted mutated tumors (5.5 v 3.3 years; HR, 0.56; 95% CI, 0.32 to 0.99; P < .05) lived longer after CRT than RT.
Conclusion: IDH mutational status identified patients with oligodendroglial tumors who did (and did not) benefit from alkylating-agent chemotherapy with RT. Although patients with codeleted tumors lived longest, patients with noncodeleted IDH-mutated tumors also lived longer after CRT.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Similar articles
-
Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402.J Clin Oncol. 2013 Jan 20;31(3):337-43. doi: 10.1200/JCO.2012.43.2674. Epub 2012 Oct 15. J Clin Oncol. 2013. PMID: 23071247 Free PMC article. Clinical Trial.
-
Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951.J Clin Oncol. 2013 Jan 20;31(3):344-50. doi: 10.1200/JCO.2012.43.2229. Epub 2012 Oct 15. J Clin Oncol. 2013. PMID: 23071237 Clinical Trial.
-
Comprehensive Genomic Analysis in NRG Oncology/RTOG 9802: A Phase III Trial of Radiation Versus Radiation Plus Procarbazine, Lomustine (CCNU), and Vincristine in High-Risk Low-Grade Glioma.J Clin Oncol. 2020 Oct 10;38(29):3407-3417. doi: 10.1200/JCO.19.02983. Epub 2020 Jul 24. J Clin Oncol. 2020. PMID: 32706640 Free PMC article.
-
Radiation and chemotherapy for high-risk lower grade gliomas: Choosing between temozolomide and PCV.Cancer Med. 2020 Jan;9(1):3-11. doi: 10.1002/cam4.2686. Epub 2019 Nov 7. Cancer Med. 2020. PMID: 31701682 Free PMC article. Review.
-
Low-grade and anaplastic oligodendroglioma.Handb Clin Neurol. 2016;134:361-80. doi: 10.1016/B978-0-12-802997-8.00022-0. Handb Clin Neurol. 2016. PMID: 26948366 Review.
Cited by
-
N6-methyladenosine-related microRNAs risk model trumps the isocitrate dehydrogenase mutation status as a predictive biomarker for the prognosis and immunotherapy in lower grade gliomas.Explor Target Antitumor Ther. 2022;3(5):553-569. doi: 10.37349/etat.2022.00100. Epub 2022 Sep 30. Explor Target Antitumor Ther. 2022. PMID: 36226036 Free PMC article.
-
Oligodendroglioma: pathology, molecular mechanisms and markers.Acta Neuropathol. 2015 Jun;129(6):809-27. doi: 10.1007/s00401-015-1424-1. Epub 2015 May 6. Acta Neuropathol. 2015. PMID: 25943885 Free PMC article. Review.
-
IDH1 and IDH2 mutations as novel therapeutic targets: current perspectives.J Blood Med. 2016 Sep 2;7:171-80. doi: 10.2147/JBM.S70716. eCollection 2016. J Blood Med. 2016. PMID: 27621679 Free PMC article. Review.
-
The Dilemma of Cure and Damage in Oligodendroglioma: Ways to Tip the Balance Away from the Damage.Cancers (Basel). 2018 Nov 12;10(11):431. doi: 10.3390/cancers10110431. Cancers (Basel). 2018. PMID: 30424475 Free PMC article.
-
Proton radiotherapy in the treatment of IDH-mutant diffuse gliomas: an early experience from shanghai proton and heavy ion center.J Neurooncol. 2023 May;162(3):503-514. doi: 10.1007/s11060-022-04202-5. Epub 2022 Dec 30. J Neurooncol. 2023. PMID: 36583815
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for patients with newly diagnosed glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. - PubMed
-
- Levin VA, Edwards MS, Wright DC, et al. Modified procarbazine, CCNU, and vincristine (PCV 3) combination chemotherapy in the treatment of malignant brain tumors. Cancer Treat Rep. 1980;64:237–244. - PubMed
-
- van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: Long-term follow-up of EORTC Brain Tumor Group study 26951. J Clin Oncol. 2013;31:344–350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 CA180868/CA/NCI NIH HHS/United States
- P50 CA108961/CA/NCI NIH HHS/United States
- RC1NS068222Z/NS/NINDS NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- U10 CA017145/CA/NCI NIH HHS/United States
- CA17145/CA/NCI NIH HHS/United States
- U10 CA37422/CA/NCI NIH HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- P30 CA015083/CA/NCI NIH HHS/United States
- U24 CA114734/CA/NCI NIH HHS/United States
- U10 CA25224/CA/NCI NIH HHS/United States
- U10CA32115/CA/NCI NIH HHS/United States
- U10 CA032115/CA/NCI NIH HHS/United States
- RC1 NS068222/NS/NINDS NIH HHS/United States
- N01 CA032102/CA/NCI NIH HHS/United States
- U10 CA037422/CA/NCI NIH HHS/United States
- P50CA108961/CA/NCI NIH HHS/United States
- U10 CA021661/CA/NCI NIH HHS/United States
- P30CA15083/CA/NCI NIH HHS/United States
- CA21115/CA/NCI NIH HHS/United States
- U10 CA025224/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- U10CA21661/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

