The role of HMGB1-RAGE axis in migration and invasion of hepatocellular carcinoma cell lines
- PMID: 24510323
- PMCID: PMC3972434
- DOI: 10.1007/s11010-014-1978-6
The role of HMGB1-RAGE axis in migration and invasion of hepatocellular carcinoma cell lines
Abstract
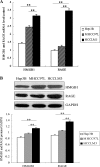
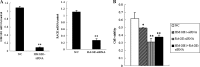
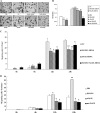
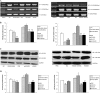
High mobility group protein box1 (HMGB1) and its receptor-receptor for advanced glycation end products (RAGE) are pivotal factors in the development and progression of many types of tumor, but the role of HMGB1-RAGE axis in hepatocellular carcinoma (HCC) especially its effects on metastasis and recurrence remains obscure. Here, we report the role of HMGB1-RAGE axis in the biological behaviors of HCC cell lines and the underlying molecular mechanism. We show that the expressions of HMGB1, RAGE, and extracellular HMGB1 increase consistently according to cell metastasis potentials, while the concentration of soluble form of RAGE (sRAGE) is inversely related to metastasis potential of HCC cells. Furthermore, our data show that rhHMGB1 promotes cellular proliferation, migration, and invasion, and increases the level of nuclear factor kappa B (NF-κB), while administrations of HMGB1-siRNA, RAGE-siRNA, anti-HMGB1 neutralizing antibody, anti-RAGE neutralizing antibody, and sRAGE inhibit cellular proliferation, migration, and invasion. Moreover, we also demonstrate that the expression of NF-кB is inhibited by knockdown of HMGB1 or RAGE. Collectively, these data demonstrate that HMGB1 activates RAGE signaling pathways and induces NF-кB activation to promote cellular proliferation, invasion, and metastasis, in HCC cell lines. Taken together, HMGB1-RAGE axis may become a potential target in HCC therapy.
Figures


Similar articles
-
HMGB1 promotes HCC progression partly by downregulating p21 via ERK/c-Myc pathway and upregulating MMP-2.Tumour Biol. 2016 Apr;37(4):4399-408. doi: 10.1007/s13277-015-4049-z. Epub 2015 Oct 24. Tumour Biol. 2016. PMID: 26499944 Free PMC article.
-
The Role of receptor for Advanced Glycation End Products (RAGE) in the proliferation of hepatocellular carcinoma.Int J Mol Sci. 2012;13(5):5982-5997. doi: 10.3390/ijms13055982. Epub 2012 May 18. Int J Mol Sci. 2012. PMID: 22754344 Free PMC article.
-
The long non-coding RNA TP73-AS1 modulates HCC cell proliferation through miR-200a-dependent HMGB1/RAGE regulation.J Exp Clin Cancer Res. 2017 Apr 12;36(1):51. doi: 10.1186/s13046-017-0519-z. J Exp Clin Cancer Res. 2017. PMID: 28403886 Free PMC article.
-
The Role of HMGB1 Signaling Pathway in the Development and Progression of Hepatocellular Carcinoma: A Review.Int J Mol Sci. 2015 Sep 17;16(9):22527-40. doi: 10.3390/ijms160922527. Int J Mol Sci. 2015. PMID: 26393575 Free PMC article. Review.
-
MicroRNA-Mediated Regulation of HMGB1 in Human Hepatocellular Carcinoma.Biomed Res Int. 2018 Jan 31;2018:2754941. doi: 10.1155/2018/2754941. eCollection 2018. Biomed Res Int. 2018. PMID: 29651425 Free PMC article. Review.
Cited by
-
Regulatory Networks, Management Approaches, and Emerging Treatments of Nonalcoholic Fatty Liver Disease.Can J Gastroenterol Hepatol. 2022 Nov 8;2022:6799414. doi: 10.1155/2022/6799414. eCollection 2022. Can J Gastroenterol Hepatol. 2022. PMID: 36397950 Free PMC article. Review.
-
Effects of RAGE Gene Polymorphisms on the Risk and Progression of Hepatocellular Carcinoma.Medicine (Baltimore). 2015 Aug;94(34):e1396. doi: 10.1097/MD.0000000000001396. Medicine (Baltimore). 2015. PMID: 26313784 Free PMC article.
-
Knockdown of HMGB1 improves apoptosis and suppresses proliferation and invasion of glioma cells.Chin J Cancer Res. 2014 Dec;26(6):658-68. doi: 10.3978/j.issn.1000-9604.2014.12.05. Chin J Cancer Res. 2014. PMID: 25561763 Free PMC article.
-
Radiotherapy-induced cell death activates paracrine HMGB1-TLR2 signaling and accelerates pancreatic carcinoma metastasis.J Exp Clin Cancer Res. 2018 Apr 3;37(1):77. doi: 10.1186/s13046-018-0726-2. J Exp Clin Cancer Res. 2018. PMID: 29615080 Free PMC article.
-
De Novo Hepatocellular Carcinoma in Hepatitis C-Related Cirrhosis: Are Advanced Glycation End Products a Key Driver?Front Cell Infect Microbiol. 2021 Oct 1;11:662431. doi: 10.3389/fcimb.2021.662431. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34660332 Free PMC article.
References
-
- Neeper M, Schmidt AM, Brett J, et al. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267:14998–15004. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

