ERCC6L2 mutations link a distinct bone-marrow-failure syndrome to DNA repair and mitochondrial function
- PMID: 24507776
- PMCID: PMC3928664
- DOI: 10.1016/j.ajhg.2014.01.007
ERCC6L2 mutations link a distinct bone-marrow-failure syndrome to DNA repair and mitochondrial function
Abstract
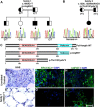
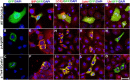
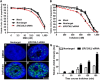
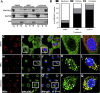
Exome sequencing was performed in three index cases with bone marrow failure and neurological dysfunction and whose parents are first-degree cousins. Homozygous truncating mutations were identified in ERCC6L2 in two of the individuals. Both of these mutations affect the subcellular localization and stability of ERCC6L2. We show here that knockdown of ERCC6L2 in human A549 cells significantly reduced their viability upon exposure to the DNA-damaging agents mitomycin C and Irofulven, but not etoposide and camptothecin, suggesting a role in nucleotide excision repair. ERCC6L2-knockdown cells also displayed H2AX phosphorylation, which significantly increased upon genotoxic stress, suggesting an early DNA-damage response. Intriguingly, ERCC6L2 was seen to translocate to the mitochondria and the nucleus in response to DNA damage, and ERCC6L2 knockdown induced intracellular reactive oxygen species (ROS). Treatment with the ROS scavenger N-acetyl cysteine attenuated the Irofulven-induced cytotoxicity in ERCC6L2-knockdown cells and abolished ERCCGL2 traffic to the mitochondria and nucleus in response to this DNA-damaging agent. Collectively, these observations identify a distinct bone-marrow-failure syndrome due to mutations in ERCC6L2, a gene implicated in DNA repair and mitochondrial function.
Copyright © 2014 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
ERCC6L2-related disease: a novel entity of bone marrow failure disorder with high risk of clonal evolution.Ann Hematol. 2023 Apr;102(4):699-705. doi: 10.1007/s00277-023-05128-2. Epub 2023 Feb 15. Ann Hematol. 2023. PMID: 36790458 Free PMC article. Review.
-
Bone marrow failure syndrome caused by homozygous frameshift mutation in the ERCC6L2 gene.Clin Genet. 2018 Feb;93(2):392-395. doi: 10.1111/cge.13125. Epub 2017 Dec 18. Clin Genet. 2018. PMID: 28815563
-
ERCC6L2-associated inherited bone marrow failure syndrome.Mol Genet Genomic Med. 2018 May;6(3):463-468. doi: 10.1002/mgg3.388. Epub 2018 Apr 6. Mol Genet Genomic Med. 2018. PMID: 29633571 Free PMC article. Review.
-
Genome instability is a consequence of transcription deficiency in patients with bone marrow failure harboring biallelic ERCC6L2 variants.Proc Natl Acad Sci U S A. 2018 Jul 24;115(30):7777-7782. doi: 10.1073/pnas.1803275115. Epub 2018 Jul 9. Proc Natl Acad Sci U S A. 2018. PMID: 29987015 Free PMC article.
-
Germline ERCC excision repair 6 like 2 (ERCC6L2) mutations lead to impaired erythropoiesis and reshaping of the bone marrow microenvironment.Br J Haematol. 2022 Dec;199(5):754-764. doi: 10.1111/bjh.18466. Epub 2022 Sep 26. Br J Haematol. 2022. PMID: 36156210 Free PMC article.
Cited by
-
Repair of programmed DNA lesions in antibody class switch recombination: common and unique features.Genome Instab Dis. 2021;2(2):115-125. doi: 10.1007/s42764-021-00035-0. Epub 2021 Mar 26. Genome Instab Dis. 2021. PMID: 33817557 Free PMC article. Review.
-
DNA damage and repair in the hematopoietic system.Acta Biochim Biophys Sin (Shanghai). 2022 Jan 25;54(6):847-857. doi: 10.3724/abbs.2022053. Acta Biochim Biophys Sin (Shanghai). 2022. PMID: 35593466 Free PMC article. Review.
-
ERCC6L2 promotes DNA orientation-specific recombination in mammalian cells.Cell Res. 2020 Sep;30(9):732-744. doi: 10.1038/s41422-020-0328-3. Epub 2020 Apr 30. Cell Res. 2020. PMID: 32355287 Free PMC article.
-
Oxidative stress, bone marrow failure, and genome instability in hematopoietic stem cells.Int J Mol Sci. 2015 Jan 22;16(2):2366-85. doi: 10.3390/ijms16022366. Int J Mol Sci. 2015. PMID: 25622253 Free PMC article. Review.
-
ERCC6L2-related disease: a novel entity of bone marrow failure disorder with high risk of clonal evolution.Ann Hematol. 2023 Apr;102(4):699-705. doi: 10.1007/s00277-023-05128-2. Epub 2023 Feb 15. Ann Hematol. 2023. PMID: 36790458 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

