Serum amino acids profile and the beneficial effects of L-arginine or L-glutamine supplementation in dextran sulfate sodium colitis
- PMID: 24505477
- PMCID: PMC3914992
- DOI: 10.1371/journal.pone.0088335
Serum amino acids profile and the beneficial effects of L-arginine or L-glutamine supplementation in dextran sulfate sodium colitis
Abstract
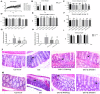
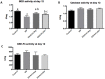
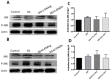
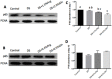
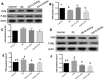
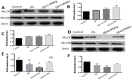
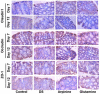
This study was conducted to investigate serum amino acids profile in dextran sulfate sodium (DSS)-induced colitis, and impacts of graded dose of arginine or glutamine supplementation on the colitis. Using DSS-induced colitis model, which is similar to human ulcerative colitis, we determined serum profile of amino acids at day 3, 7, 10 and 12 (5 days post DSS treatment). Meanwhile, effects of graded dose of arginine (0.4%, 0.8%, and 1.5%) or glutamine (0.5%, 1.0% and 2.0%) supplementation on clinical parameters, serum amino acids, colonic tight junction proteins, colonic anti-oxidative indicators [catalase, total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px)], colonic pro-inflammatory cytokines [interleukin-1 beta (IL-1β), IL-6, IL-17 and tumor necrosis factor alpha (TNF-α)] in DSS-induced colitis were fully analyzed at day 7 and 12. Additionally, the activation of signal transduction pathways, including nuclear factor kappa B (NF-κB), mitogen-activated protein kinases (MAPK), phosphoinositide-3-kinases (PI3K)/PI3K-protein kinase B (Akt), and myosin light chain kinase (MLCK)-myosin light chain (MLC20), were analyzed using immunoblotting. Serum amino acids analysis showed that DSS treatment changed the serum contents of amino acids, such as Trp, Glu, and Gln (P<0.05). Dietary arginine or glutamine supplementation had significant (P<0.05) influence on the clinical and biochemical parameters (T-SOD, IL-17 and TNF-α) in colitis model. These results were associated with colonic NF-κB, PI3K-Akt and MLCK signaling pathways. In conclusion, arginine or glutamine could be a potential therapy for intestinal inflammatory diseases.
Conflict of interest statement
Figures

Similar articles
-
Canna x generalis L.H. Bailey rhizome extract ameliorates dextran sulfate sodium-induced colitis via modulating intestinal mucosal dysfunction, oxidative stress, inflammation, and TLR4/ NF-ҡB and NLRP3 inflammasome pathways.J Ethnopharmacol. 2021 Apr 6;269:113670. doi: 10.1016/j.jep.2020.113670. Epub 2020 Dec 8. J Ethnopharmacol. 2021. PMID: 33301917
-
L-arginine supplementation improves responses to injury and inflammation in dextran sulfate sodium colitis.PLoS One. 2012;7(3):e33546. doi: 10.1371/journal.pone.0033546. Epub 2012 Mar 12. PLoS One. 2012. PMID: 22428068 Free PMC article.
-
Effects of dietary glutamine on the homeostasis of CD4+ T cells in mice with dextran sulfate sodium-induced acute colitis.PLoS One. 2014 Jan 9;9(1):e84410. doi: 10.1371/journal.pone.0084410. eCollection 2014. PLoS One. 2014. PMID: 24416230 Free PMC article.
-
Oral administration of the flavonoid myricitrin prevents dextran sulfate sodium-induced experimental colitis in mice through modulation of PI3K/Akt signaling pathway.Mol Nutr Food Res. 2013 Nov;57(11):1938-49. doi: 10.1002/mnfr.201300134. Epub 2013 Jul 17. Mol Nutr Food Res. 2013. PMID: 23861337
-
Protective effects of panax notoginseng saponin on dextran sulfate sodium-induced colitis in rats through phosphoinositide-3-kinase protein kinase B signaling pathway inhibition.World J Gastroenterol. 2020 Mar 21;26(11):1156-1171. doi: 10.3748/wjg.v26.i11.1156. World J Gastroenterol. 2020. PMID: 32231420 Free PMC article.
Cited by
-
Animal Models and Pathogenesis of Ulcerative Colitis.Comput Math Methods Med. 2022 Jul 11;2022:5927384. doi: 10.1155/2022/5927384. eCollection 2022. Comput Math Methods Med. 2022. Retraction in: Comput Math Methods Med. 2023 Jun 28;2023:9878915. doi: 10.1155/2023/9878915 PMID: 35860188 Free PMC article. Retracted.
-
Versatile Nutraceutical Potentials of Watermelon-A Modest Fruit Loaded with Pharmaceutically Valuable Phytochemicals.Molecules. 2020 Nov 11;25(22):5258. doi: 10.3390/molecules25225258. Molecules. 2020. PMID: 33187365 Free PMC article. Review.
-
Role of the microbiome and its metabolites in ankylosing spondylitis.Front Immunol. 2022 Oct 13;13:1010572. doi: 10.3389/fimmu.2022.1010572. eCollection 2022. Front Immunol. 2022. PMID: 36311749 Free PMC article. Review.
-
Inhibiting protein arginine deiminases has antioxidant consequences.J Pharmacol Exp Ther. 2015 Apr;353(1):64-70. doi: 10.1124/jpet.115.222745. Epub 2015 Jan 29. J Pharmacol Exp Ther. 2015. PMID: 25635139 Free PMC article.
-
Korean Traditional Medicine (Jakyakgamcho-tang) Ameliorates Colitis by Regulating Gut Microbiota.Metabolites. 2019 Oct 14;9(10):226. doi: 10.3390/metabo9100226. Metabolites. 2019. PMID: 31615012 Free PMC article.
References
-
- Cho JH (2008) The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol 8: 458–466. - PubMed
-
- Rutgeerts P, Vermeire S, Van Assche G (2009) Biological therapies for inflammatory bowel diseases. Gastroenterology 136: 1182–1197. - PubMed
-
- Verlinden L, Leyssens C, Beullens I, Marcelis S, Mathieu C, et al... (2012) The vitamin D analog TX527 ameliorates disease symptoms in a chemically induced model of inflammatory bowel disease. J Steroid Biochem Mol Biol. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

