CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8+ T cells
- PMID: 24498562
- PMCID: PMC3902121
- DOI: 10.4161/onci.26968
CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8+ T cells
Abstract
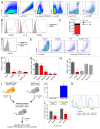
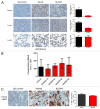
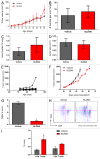
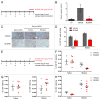
Increased numbers of tumor-infiltrating macrophages correlate with poor disease outcome in patients affected by several types of cancer, including breast and prostate carcinomas. The colony stimulating factor 1 receptor (CSF1R) signaling pathway drives the recruitment of tumor-associated macrophages (TAMs) to the neoplastic microenvironment and promotes the differentiation of TAMs toward a pro-tumorigenic phenotype. Twelve clinical trials are currently evaluating agents that target the CSF1/CSF1R signaling pathway as a treatment against multiple malignancies, including breast carcinoma, leukemia, and glioblastoma. The blockade of CSF1R signaling has been shown to greatly decrease the number of macrophages in a tissue-specific manner. However, additional mechanistic insights are needed in order to understand how macrophages are depleted and the global effects of CSF1R inhibition on other tumor-infiltrating immune cells. Using BLZ945, a highly selective small molecule inhibitor of CSF1R, we show that CSF1R inhibition attenuates the turnover rate of TAMs while increasing the number of CD8+ T cells that infiltrate cervical and breast carcinomas. Specifically, we find that BLZ945 decreased the growth of malignant cells in the mouse mammary tumor virus-driven polyomavirus middle T antigen (MMTV-PyMT) model of mammary carcinogenesis. Furthermore, we show that BLZ945 prevents tumor progression in the keratin 14-expressing human papillomavirus type 16 (K14-HPV-16) transgenic model of cervical carcinogenesis. Our results demonstrate that TAMs undergo a constant turnover in a CSF1R-dependent manner, and suggest that continuous inhibition of the CSF1R pathway may be essential to maintain efficacious macrophage depletion as an anticancer therapy.
Keywords: CSF1R; M-CSF; breast cancer; cervical cancer; transgenic mouse models; tumor immune evasion; tumor immunology; tumor-associated macrophages.
Figures
Similar articles
-
CSF1/CSF1R Axis Blockade Limits Mesothelioma and Enhances Efficiency of Anti-PDL1 Immunotherapy.Cancers (Basel). 2021 May 22;13(11):2546. doi: 10.3390/cancers13112546. Cancers (Basel). 2021. PMID: 34067348 Free PMC article.
-
Disruption of tumour-associated macrophage trafficking by the osteopontin-induced colony-stimulating factor-1 signalling sensitises hepatocellular carcinoma to anti-PD-L1 blockade.Gut. 2019 Sep;68(9):1653-1666. doi: 10.1136/gutjnl-2019-318419. Epub 2019 Mar 22. Gut. 2019. PMID: 30902885
-
Dual inhibition of CSF1R and MAPK pathways using supramolecular nanoparticles enhances macrophage immunotherapy.Biomaterials. 2020 Jan;227:119559. doi: 10.1016/j.biomaterials.2019.119559. Epub 2019 Oct 19. Biomaterials. 2020. PMID: 31670078 Free PMC article.
-
Small-Molecule CSF1R Inhibitors as Anticancer Agents.Curr Med Chem. 2020;27(23):3944-3966. doi: 10.2174/1573394715666190618121649. Curr Med Chem. 2020. PMID: 31215373 Review.
-
CSF1R inhibitors are emerging immunotherapeutic drugs for cancer treatment.Eur J Med Chem. 2023 Jan 5;245(Pt 1):114884. doi: 10.1016/j.ejmech.2022.114884. Epub 2022 Oct 29. Eur J Med Chem. 2023. PMID: 36335744 Review.
Cited by
-
Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils.Proc Natl Acad Sci U S A. 2015 Feb 10;112(6):E566-75. doi: 10.1073/pnas.1424927112. Epub 2015 Jan 26. Proc Natl Acad Sci U S A. 2015. PMID: 25624500 Free PMC article.
-
Leukaemia exposure alters the transcriptional profile and function of BCR::ABL1 negative macrophages in the bone marrow niche.Nat Commun. 2024 Feb 5;15(1):1090. doi: 10.1038/s41467-024-45471-0. Nat Commun. 2024. PMID: 38316788 Free PMC article.
-
Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy.Nat Med. 2016 Aug;22(8):851-60. doi: 10.1038/nm.4123. Epub 2016 Jul 4. Nat Med. 2016. PMID: 27376576 Free PMC article.
-
CD14+ macrophage-like cells as the linchpin of cervical cancer perpetrated immune suppression and early metastatic spread: A new therapeutic lead?Oncoimmunology. 2015 May 7;4(6):e1009296. doi: 10.1080/2162402X.2015.1009296. eCollection 2015 Jun. Oncoimmunology. 2015. PMID: 26155430 Free PMC article.
-
Targeting tumor-associated macrophages to combat pancreatic cancer.Oncotarget. 2016 Aug 2;7(31):50735-50754. doi: 10.18632/oncotarget.9383. Oncotarget. 2016. PMID: 27191744 Free PMC article. Review.
References
-
- Solinas G, Schiarea S, Liguori M, Fabbri M, Pesce S, Zammataro L, Pasqualini F, Nebuloni M, Chiabrando C, Mantovani A, et al. Tumor-conditioned macrophages secrete migration-stimulating factor: a new marker for M2-polarization, influencing tumor cell motility. J Immunol. 2010;185:642–52. doi: 10.4049/jimmunol.1000413. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
