Neuro-anatomical evidence indicating indirect modulation of macrophages by vagal efferents in the intestine but not in the spleen
- PMID: 24489965
- PMCID: PMC3906221
- DOI: 10.1371/journal.pone.0087785
Neuro-anatomical evidence indicating indirect modulation of macrophages by vagal efferents in the intestine but not in the spleen
Abstract
Background: Electrical stimulation of the vagus nerve suppresses intestinal inflammation and normalizes gut motility in a mouse model of postoperative ileus. The exact anatomical interaction between the vagus nerve and the intestinal immune system remains however a matter of debate. In the present study, we provide additional evidence on the direct and indirect vagal innervation of the spleen and analyzed the anatomical evidence for neuroimmune modulation of macrophages by vagal preganglionic and enteric postganglionic nerve fibers within the intestine.
Methods: Dextran conjugates were used to label vagal preganglionic (motor) fibers projecting to the small intestine and spleen. Moreover, identification of the neurochemical phenotype of the vagal efferent fibers and enteric neurons was performed by immunofluorescent labeling. F4/80 antibody was used to label resident macrophages.
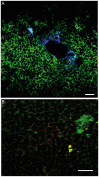
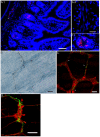
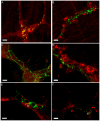
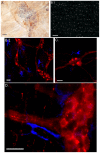
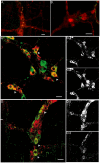
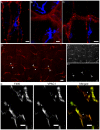
Results: Our anterograde tracing experiments did not reveal dextran-labeled vagal fibers or terminals in the mesenteric ganglion or spleen. Vagal efferent fibers were confined within the myenteric plexus region of the small intestine and mainly endings around nNOS, VIP and ChAT positive enteric neurons. nNOS, VIP and ChAT positive fibers were found in close proximity of intestinal resident macrophages carrying α7 nicotinic receptors. Of note, VIP receptors were found on resident macrophages located in close proximity of VIP positive nerve fibers.
Conclusion: In the present study, we show that the vagus nerve does not directly interact with resident macrophages in the gut or spleen. Instead, the vagus nerve preferentially interacts with nNOS, VIP and ChAT enteric neurons located within the gut muscularis with nerve endings in close proximity of the resident macrophages.
Conflict of interest statement
Figures


Similar articles
-
A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen.Gut. 2014 Jun;63(6):938-48. doi: 10.1136/gutjnl-2013-304676. Epub 2013 Aug 8. Gut. 2014. PMID: 23929694
-
Identification of vagal efferent fibers and putative target neurons in the enteric nervous system of the rat.J Comp Neurol. 1989 Jul 1;285(1):38-53. doi: 10.1002/cne.902850105. J Comp Neurol. 1989. PMID: 2568999
-
Vagal preganglionic axons arborize in the myenteric plexus into two types: nitrergic and non-nitrergic postganglionic motor pools?Am J Physiol Regul Integr Comp Physiol. 2023 Mar 1;324(3):R305-R316. doi: 10.1152/ajpregu.00260.2022. Epub 2023 Jan 9. Am J Physiol Regul Integr Comp Physiol. 2023. PMID: 36622086 Free PMC article.
-
Musings on the wanderer: what's new in our understanding of vago-vagal reflex? IV. Current concepts of vagal efferent projections to the gut.Am J Physiol Gastrointest Liver Physiol. 2003 Mar;284(3):G357-66. doi: 10.1152/ajpgi.00478.2002. Am J Physiol Gastrointest Liver Physiol. 2003. PMID: 12576302 Review.
-
Vagal Modulation of the Inflammatory Response in Sepsis.Int Rev Immunol. 2016 Sep 2;35(5):415-433. doi: 10.3109/08830185.2015.1127369. Epub 2016 Apr 29. Int Rev Immunol. 2016. PMID: 27128144 Review.
Cited by
-
Ion channel regulation of gut immunity.J Gen Physiol. 2023 Feb 6;155(2):e202113042. doi: 10.1085/jgp.202113042. Epub 2022 Dec 2. J Gen Physiol. 2023. PMID: 36459135 Free PMC article.
-
Type 3 Muscarinic Receptors Contribute to Clearance of Citrobacter rodentium.Inflamm Bowel Dis. 2015 Aug;21(8):1860-71. doi: 10.1097/MIB.0000000000000408. Inflamm Bowel Dis. 2015. PMID: 25985244 Free PMC article.
-
Recent advances in understanding the roles of the enteric nervous system.Fac Rev. 2022 Mar 24;11:7. doi: 10.12703/r/11-7. eCollection 2022. Fac Rev. 2022. PMID: 35373214 Free PMC article. Review.
-
Eating for 3.8 × 1013: Examining the Impact of Diet and Nutrition on the Microbiota-Gut-Brain Axis Through the Lens of Microbial Endocrinology.Front Endocrinol (Lausanne). 2019 Jan 29;9:796. doi: 10.3389/fendo.2018.00796. eCollection 2018. Front Endocrinol (Lausanne). 2019. PMID: 30761092 Free PMC article. Review.
-
Regulation of the Autonomic Nervous System on Intestine.Front Physiol. 2021 Jul 14;12:700129. doi: 10.3389/fphys.2021.700129. eCollection 2021. Front Physiol. 2021. PMID: 34335306 Free PMC article. Review.
References
-
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, et al. (2000) Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405: 458–462 10.1038/35013070 [doi]. - PubMed
-
- Tracey KJ (2002) The inflammatory reflex. Nature 420: 853–859 10.1038/nature01321 [doi];nature01321 [pii]. - PubMed
-
- Bernik TR, Friedman SG, Ochani M, DiRaimo R, Susarla S, et al. (2002) Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J Vasc Surg 36: 1231–1236 10.1067/mva.2002.129643 [doi];S0741521402003208 [pii]. - PubMed
-
- Guarini S, Altavilla D, Cainazzo MM, Giuliani D, Bigiani A, et al. (2003) Efferent vagal fibre stimulation blunts nuclear factor-kappaB activation and protects against hypovolemic hemorrhagic shock. Circulation 107: 1189–1194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

