Long-lived intestinal tuft cells serve as colon cancer-initiating cells
- PMID: 24487592
- PMCID: PMC3934168
- DOI: 10.1172/JCI73434
Long-lived intestinal tuft cells serve as colon cancer-initiating cells
Abstract
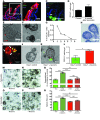
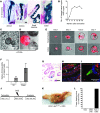
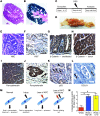
Doublecortin-like kinase 1 protein (DCLK1) is a gastrointestinal tuft cell marker that has been proposed to identify quiescent and tumor growth-sustaining stem cells. DCLK1⁺ tuft cells are increased in inflammation-induced carcinogenesis; however, the role of these cells within the gastrointestinal epithelium and their potential as cancer-initiating cells are poorly understood. Here, using a BAC-CreERT-dependent genetic lineage-tracing strategy, we determined that a subpopulation of DCLK1⁺ cells is extremely long lived and possesses rare stem cell abilities. Moreover, genetic ablation of Dclk1 revealed that DCLK1⁺ tuft cells contribute to recovery following intestinal and colonic injury. Surprisingly, conditional knockdown of the Wnt regulator APC in DCLK1⁺ cells was not sufficient to drive colonic carcinogenesis under normal conditions; however, dextran sodium sulfate-induced (DSS-induced) colitis promoted the development of poorly differentiated colonic adenocarcinoma in mice lacking APC in DCLK1⁺ cells. Importantly, colonic tumor formation occurred even when colitis onset was delayed for up to 3 months after induced APC loss in DCLK1⁺ cells. Thus, our data define an intestinal DCLK1⁺ tuft cell population that is long lived, quiescent, and important for intestinal homeostasis and regeneration. Long-lived DCLK1⁺ cells maintain quiescence even following oncogenic mutation, but are activated by tissue injury and can serve to initiate colon cancer.
Figures


Similar articles
-
Dclk1, a tumor stem cell marker, regulates pro-survival signaling and self-renewal of intestinal tumor cells.Mol Cancer. 2017 Feb 1;16(1):30. doi: 10.1186/s12943-017-0594-y. Mol Cancer. 2017. PMID: 28148261 Free PMC article.
-
Ablation of Doublecortin-Like Kinase 1 in the Colonic Epithelium Exacerbates Dextran Sulfate Sodium-Induced Colitis.PLoS One. 2015 Aug 18;10(8):e0134212. doi: 10.1371/journal.pone.0134212. eCollection 2015. PLoS One. 2015. PMID: 26285154 Free PMC article.
-
Dclk1 in tuft cells promotes inflammation-driven epithelial restitution and mitigates chronic colitis.Cell Death Differ. 2019 Sep;26(9):1656-1669. doi: 10.1038/s41418-018-0237-x. Epub 2018 Nov 26. Cell Death Differ. 2019. PMID: 30478383 Free PMC article.
-
Functional implication of Dclk1 and Dclk1-expressing cells in cancer.Small GTPases. 2017 Jul 3;8(3):164-171. doi: 10.1080/21541248.2016.1208792. Epub 2016 Jul 26. Small GTPases. 2017. PMID: 27458755 Free PMC article. Review.
-
Dclk1-expressing tuft cells: critical modulators of the intestinal niche?Am J Physiol Gastrointest Liver Physiol. 2017 Oct 1;313(4):G285-G299. doi: 10.1152/ajpgi.00073.2017. Epub 2017 Jul 6. Am J Physiol Gastrointest Liver Physiol. 2017. PMID: 28684459 Free PMC article. Review.
Cited by
-
Emerging roles for IL-25 and IL-33 in colorectal cancer tumorigenesis.Front Immunol. 2022 Oct 3;13:981479. doi: 10.3389/fimmu.2022.981479. eCollection 2022. Front Immunol. 2022. PMID: 36263033 Free PMC article. Review.
-
Epigenetic regulation of human DCLK-1 gene during colon-carcinogenesis: clinical and mechanistic implications.Stem Cell Investig. 2016 Sep 28;3:51. doi: 10.21037/sci.2016.09.07. eCollection 2016. Stem Cell Investig. 2016. PMID: 27777940 Free PMC article.
-
Epithelial wound healing in inflammatory bowel diseases: the next therapeutic frontier.Transl Res. 2021 Oct;236:35-51. doi: 10.1016/j.trsl.2021.06.001. Epub 2021 Jun 12. Transl Res. 2021. PMID: 34126257 Free PMC article. Review.
-
Environmental Impact on Intestinal Stem Cell Functions in Mucosal Homeostasis and Tumorigenesis.J Cell Biochem. 2017 May;118(5):943-952. doi: 10.1002/jcb.25719. Epub 2017 Jan 11. J Cell Biochem. 2017. PMID: 27584938 Free PMC article. Review.
-
Goblet Cell Ratio in Combination with Differentiation and Stem Cell Markers in Barrett Esophagus Allow Distinction of Patients with and without Esophageal Adenocarcinoma.Cancer Prev Res (Phila). 2017 Jan;10(1):55-66. doi: 10.1158/1940-6207.CAPR-16-0117. Epub 2016 Nov 2. Cancer Prev Res (Phila). 2017. PMID: 27807078 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

