Hedgehog acyltransferase as a target in pancreatic ductal adenocarcinoma
- PMID: 24469057
- PMCID: PMC4513646
- DOI: 10.1038/onc.2013.575
Hedgehog acyltransferase as a target in pancreatic ductal adenocarcinoma
Abstract
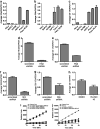
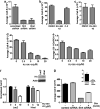
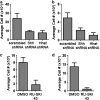
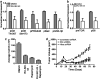
Sonic Hedgehog (Shh) is abnormally expressed in pancreatic cancer and is associated with disease onset and progression. Inhibition of Shh signaling is thus an attractive clinical target for therapeutic intervention. Most efforts to block Shh signaling have focused on inhibitors of Smoothened, which target the canonical Shh signaling pathway. These approaches have met with limited success, in part due to development of resistance-conferring mutations and contributions from non-canonical signaling pathways. Here, we show that Hedgehog acyltransferase (Hhat), the enzyme responsible for the attachment of palmitate onto Shh, is a novel target for inhibition of Shh signaling in pancreatic cancer cells. Depletion of Hhat with lentivirally delivered small hairpin RNA decreased both anchorage-dependent and independent proliferation of human pancreatic cancer cells. In vivo, Hhat knockdown led to reduction of tumor growth in a mouse xenograft model of pancreatic cancer. RU-SKI 43, a small molecule inhibitor of Hhat recently developed by our group, reduced pancreatic cancer cell proliferation and Gli-1 activation through Smoothened-independent non-canonical signaling. In addition, RU-SKI 43 treatment inhibited two key proliferative pathways regulated by Akt and mTOR. This work demonstrates that Hhat has a critical role in pancreatic cancer and that a small molecule inhibitor of Hhat can successfully block pancreatic cancer cell proliferation. It also highlights the importance of developing optimized Hhat inhibitors to be used as therapeutics in pancreatic cancer, as well as in other malignancies characterized by Shh overexpression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Hedgehog Acyltransferase as a target in estrogen receptor positive, HER2 amplified, and tamoxifen resistant breast cancer cells.Mol Cancer. 2015 Apr 1;14:72. doi: 10.1186/s12943-015-0345-x. Mol Cancer. 2015. PMID: 25889650 Free PMC article.
-
Attenuation of hedgehog acyltransferase-catalyzed sonic Hedgehog palmitoylation causes reduced signaling, proliferation and invasiveness of human carcinoma cells.PLoS One. 2014 Mar 7;9(3):e89899. doi: 10.1371/journal.pone.0089899. eCollection 2014. PLoS One. 2014. PMID: 24608521 Free PMC article.
-
Characterization of Hedgehog Acyltransferase Inhibitors Identifies a Small Molecule Probe for Hedgehog Signaling by Cancer Cells.ACS Chem Biol. 2016 Dec 16;11(12):3256-3262. doi: 10.1021/acschembio.6b00896. Epub 2016 Oct 25. ACS Chem Biol. 2016. PMID: 27779865 Free PMC article.
-
Palmitoylation of Hedgehog proteins by Hedgehog acyltransferase: roles in signalling and disease.Open Biol. 2021 Mar;11(3):200414. doi: 10.1098/rsob.200414. Epub 2021 Mar 3. Open Biol. 2021. PMID: 33653085 Free PMC article. Review.
-
Shh signaling and pancreatic cancer: implications for therapy?Cell Cycle. 2007 Jul 1;6(13):1553-7. doi: 10.4161/cc.6.13.4467. Epub 2007 May 18. Cell Cycle. 2007. PMID: 17611415 Review.
Cited by
-
Current Status of Hedgehog Signaling Inhibitors.Curr Top Med Chem. 2024;24(3):243-258. doi: 10.2174/0115680266280850231221074340. Curr Top Med Chem. 2024. PMID: 38231069 Review.
-
Safety and Tolerability of Sonic Hedgehog Pathway Inhibitors in Cancer.Drug Saf. 2019 Feb;42(2):263-279. doi: 10.1007/s40264-018-0777-5. Drug Saf. 2019. PMID: 30649745 Free PMC article. Review.
-
Substrate and product complexes reveal mechanisms of Hedgehog acylation by HHAT.Science. 2021 Jun 11;372(6547):1215-1219. doi: 10.1126/science.abg4998. Science. 2021. PMID: 34112694 Free PMC article.
-
Membrane topology of hedgehog acyltransferase.J Biol Chem. 2015 Jan 23;290(4):2235-43. doi: 10.1074/jbc.M114.625764. Epub 2014 Dec 8. J Biol Chem. 2015. PMID: 25488661 Free PMC article.
-
NKX6-1 mediates cancer stem-like properties and regulates sonic hedgehog signaling in leiomyosarcoma.J Biomed Sci. 2021 Apr 27;28(1):32. doi: 10.1186/s12929-021-00726-6. J Biomed Sci. 2021. PMID: 33906647 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

