Generation of folliculogenic human epithelial stem cells from induced pluripotent stem cells
- PMID: 24468981
- PMCID: PMC4049184
- DOI: 10.1038/ncomms4071
Generation of folliculogenic human epithelial stem cells from induced pluripotent stem cells
Abstract
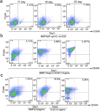
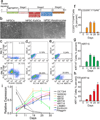
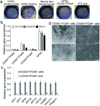
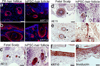
Epithelial stem cells (EpSCs) in the hair follicle bulge are required for hair follicle growth and cycling. The isolation and propagation of human EpSCs for tissue engineering purposes remains a challenge. Here we develop a strategy to differentiate human iPSCs (hiPSCs) into CD200(+)/ITGA6(+) EpSCs that can reconstitute the epithelial components of the hair follicle and interfollicular epidermis. The hiPSC-derived CD200(+)/ITGA6(+) cells show a similar gene expression signature as EpSCs directly isolated from human hair follicles. Human iPSC-derived CD200(+)/ITGA6(+) cells are capable of generating all hair follicle lineages including the hair shaft, and the inner and outer root sheaths in skin reconstitution assays. The regenerated hair follicles possess a KRT15(+) stem cell population and produce hair shafts expressing hair-specific keratins. These results suggest an approach for generating large numbers of human EpSCs for tissue engineering and new treatments for hair loss, wound healing and other degenerative skin disorders.
Figures


Similar articles
-
Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200-rich and CD34-positive hair follicle progenitor cells.J Clin Invest. 2011 Feb;121(2):613-22. doi: 10.1172/JCI44478. Epub 2011 Jan 4. J Clin Invest. 2011. PMID: 21206086 Free PMC article.
-
Feasibility of repairing full-thickness skin defects by iPSC-derived epithelial stem cells seeded on a human acellular amniotic membrane.Stem Cell Res Ther. 2019 May 31;10(1):155. doi: 10.1186/s13287-019-1234-9. Stem Cell Res Ther. 2019. PMID: 31151466 Free PMC article.
-
Ovine Hair Follicle Stem Cells Derived from Single Vibrissae Reconstitute Haired Skin.Int J Mol Sci. 2015 Aug 3;16(8):17779-97. doi: 10.3390/ijms160817779. Int J Mol Sci. 2015. PMID: 26247934 Free PMC article.
-
Epithelial stem cells: a folliculocentric view.J Invest Dermatol. 2006 Jul;126(7):1459-68. doi: 10.1038/sj.jid.5700376. J Invest Dermatol. 2006. PMID: 16778814 Review.
-
Hair follicle bulge: a fascinating reservoir of epithelial stem cells.J Dermatol Sci. 2007 May;46(2):81-9. doi: 10.1016/j.jdermsci.2006.12.002. Epub 2007 Jan 5. J Dermatol Sci. 2007. PMID: 17207970 Review.
Cited by
-
Status of research on the development and regeneration of hair follicles.Int J Med Sci. 2024 Jan 1;21(1):80-94. doi: 10.7150/ijms.88508. eCollection 2024. Int J Med Sci. 2024. PMID: 38164355 Free PMC article. Review.
-
Smurf2-induced degradation of SMAD2 causes inhibition of hair follicle stem cell differentiation.Cell Death Discov. 2022 Apr 4;8(1):160. doi: 10.1038/s41420-022-00920-x. Cell Death Discov. 2022. PMID: 35379779 Free PMC article.
-
Progress and Future Prospectives in Skin-on-Chip Development with Emphasis on the use of Different Cell Types and Technical Challenges.Stem Cell Rev Rep. 2017 Jun;13(3):418-429. doi: 10.1007/s12015-017-9737-1. Stem Cell Rev Rep. 2017. PMID: 28536890 Free PMC article. Review.
-
Simple approach to three-color two-photon microscopy by a fiber-optic wavelength convertor.Biomed Opt Express. 2016 Oct 31;7(11):4803-4815. doi: 10.1364/BOE.7.004803. eCollection 2016 Nov 1. Biomed Opt Express. 2016. PMID: 27896017 Free PMC article.
-
Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration.Pharmaceutics. 2020 Aug 5;12(8):735. doi: 10.3390/pharmaceutics12080735. Pharmaceutics. 2020. PMID: 32764269 Free PMC article. Review.
References
-
- Morris RJ, et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. - PubMed
-
- Ito M, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. - PubMed
-
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

